Improving Water Resistance of Water-Based Coatings Using Reactive Surfactants, Part 4

Davizro / iStock / Getty Images Plus, via Getty Images.
Continuing from the previous three articles, this month we will look at additional polymerization processes, how the reactive non-ionic surfactant was incorporated, how it affected the properties of the emulsion, and make some conclusions. While the results from the previous article’s process were good, we wanted to see if we could improve the properties or reduce the amount of surfactant used.
Process Two
Process two was based on a process previously studied by the group of Professor Asua for maximizing the incorporation of reactive surfactants in formulations that use a combination of a conventional surfactant and a reactive surfactant.2, 3
This process uses two steps. In the first step, there is the generation of seeds with particle size in the range of 40 to 50 nm using only the conventional surfactant. In the second step, there is the growth of a defined number of seeds using only the reactive surfactant.
This process is interesting because it favors the migration and adsorption of the reactive surfactant on the surface of the growing seeds, thus increasing its probability of reacting with the main monomers and staying on the particle surface. Based on literature, this process allowed us to generate final emulsions with average particle size in the range of 120 to 170 nm while using low content of reactive surfactants, in the range of 1 to 3 phm.2,3
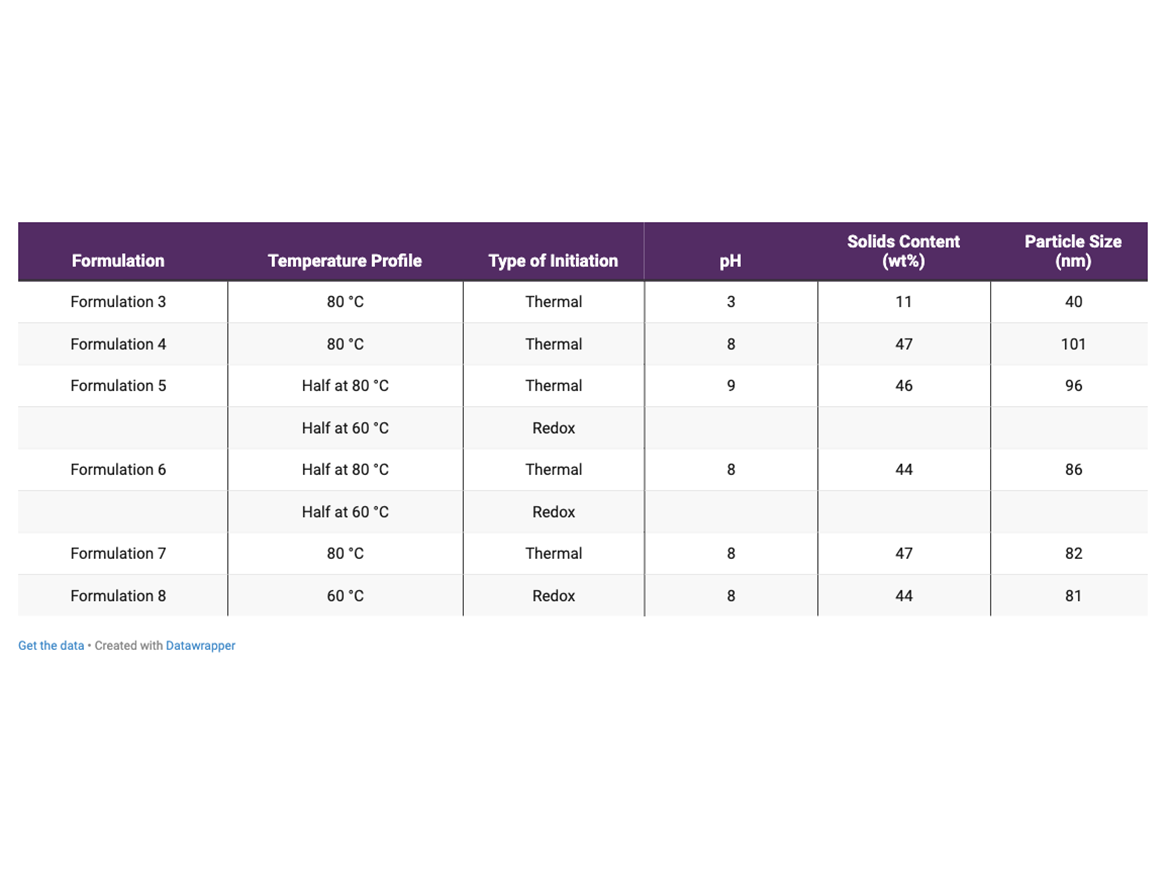
In this work, the seeds of styrene-acrylic latex were generated using only a conventional anionic surfactant based on lauryl ether sulfate. This formulation is named as Formulation 3. The content of anionic surfactant required for generating seeds of about 40 nm was 25 phm. The properties of these seeds are presented in Table 1.
In the second step, approximately 1018 seeds/L were charged in the reactor and grown through continuous additions of monomer mixture containing 2 phm of REACT N1, and initiator solution over 4 and 4.5 hours, respectively.
Formulations 4 and 5 were prepared using only REACT N1 as stabilizer in the second step. Formulation 4 was performed using thermal initiator at 80 °C, while Formulation 5 was performed half at 80 °C using thermal initiator and half at 60 °C using redox initiator. The main properties of these emulsions are also presented in Table 1.
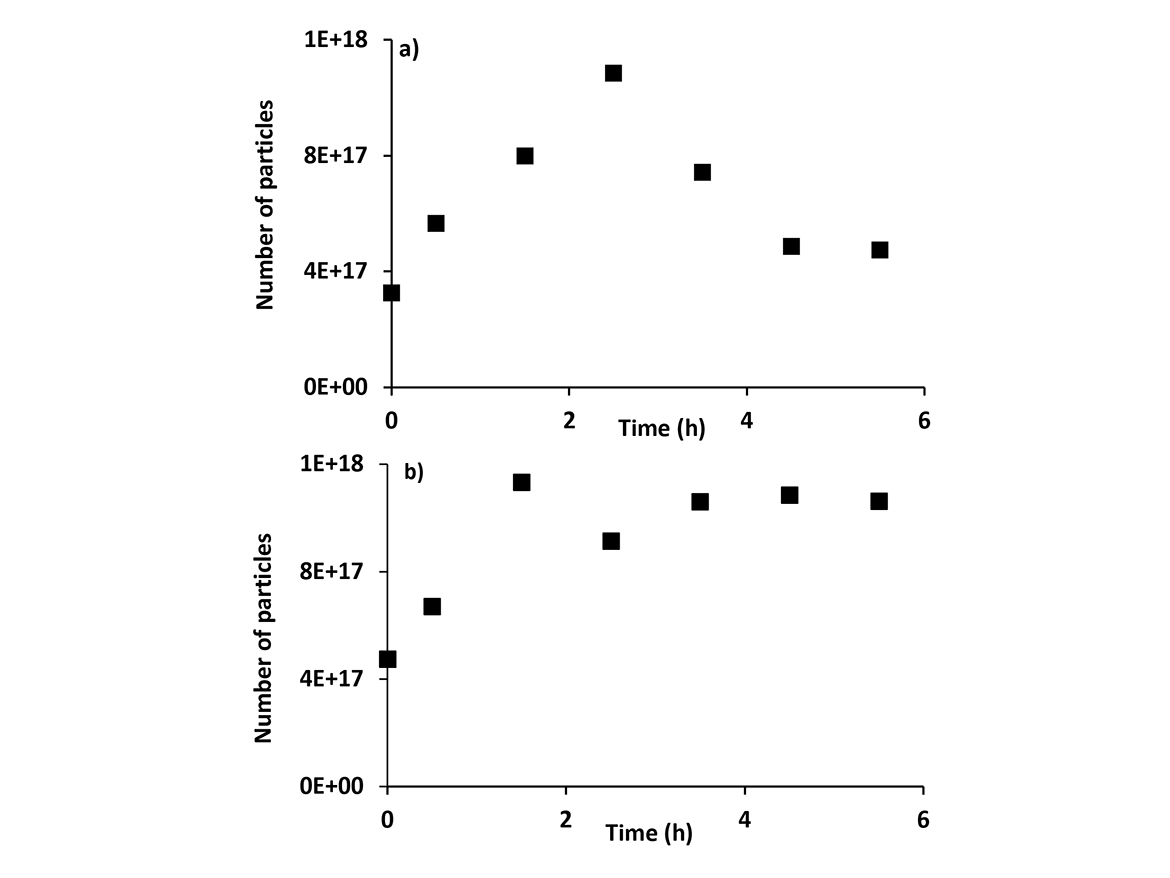
Both Formulations 4 and 5 generated more coagulum in the reactor than observed in formulations performed according to Process 1. Moreover, the plot of number of particles as a function of time of polymerization of Formulation 5 (Figure 1a), showed that there is an increase in the number of particles during the first 2.5 hours of polymerization, followed by a decrease in the number of particles. The polymerization time of 2.5 hours corresponded to 2 hours of addition of the monomer mixture containing REACT N1. Assuming that this decrease in the number of particles was the main reason for the higher coagulum formation in the reactor observed in Formulations 4 and 5, it was performed post-addition of conventional anionic surfactant in the following polymerizations in order to increase the surfactant coverage, improve the stability of latex particles, and mitigate their partial coagulation.
Formulations 6 to 8 were performed with a post-addition of 0.4 phm of the conventional anionic surfactant after 2 hours of addition of the monomer mixture. Figure 1b shows the effect of this post-addition of conventional anionic surfactant on the evolution of particle number of Formulation 6. These results showed that post-addition of the conventional anionic surfactant mitigated the partial coagulation of latex particles.
The general properties of latex from Formulation 6 are also presented in Table 1. An interesting feature of the emulsions generated from formulations with post-addition of anionic surfactant is their particle size in the range of 80 to 90 nm.
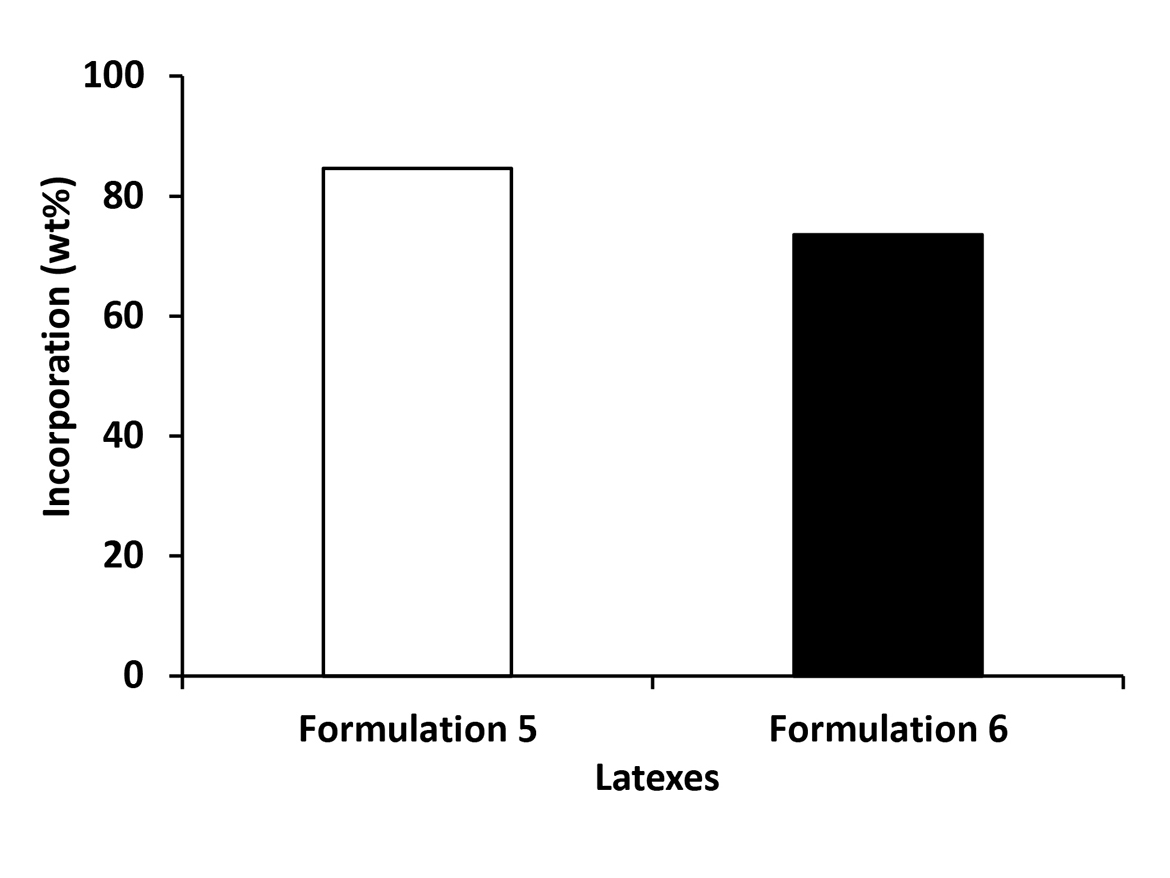
The effect of post-addition of conventional anionic surfactant on incorporation of REACT N1 in emulsion particles from Formulation 6 is presented in Figure 2. The latex from Formulation 6 presented lower incorporation of REACT N1, probably due to its higher anionic surfactant coverage, in relation to the latex from Formulation 5.
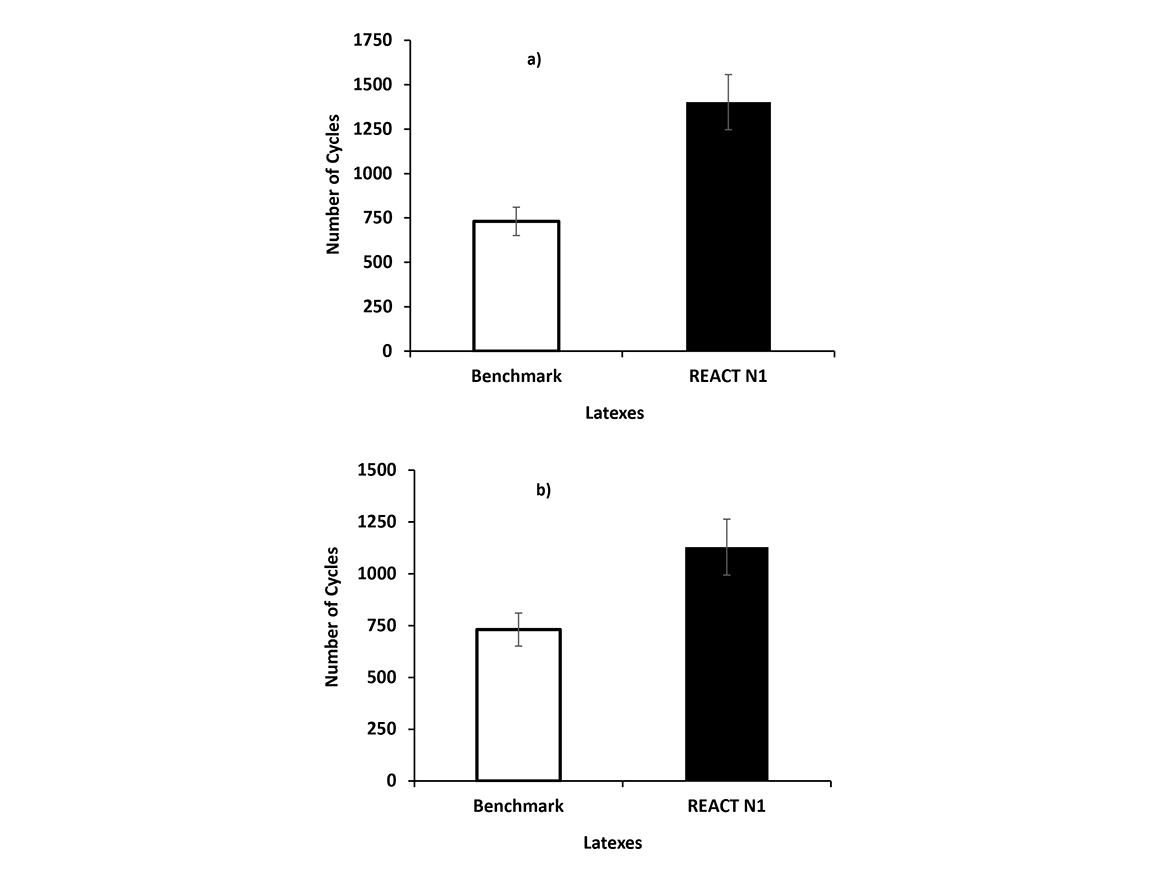
The wet scrub resistance of paints formulated with emulsions from Formulations 5 and 6 is presented in Figure 3. The paint formulated with Formulation 5 emulsion had a wet scrub resistance 92% higher than the benchmark, while the paint formulated with latex from Formulation 6 presented wet scrub resistance 54% higher than the benchmark. These results showed the expected balance between increase of stability of the latex particles caused by the post addition of anionic surfactant in the latex from Formulation 6 and the decrease in the water resistance of final coatings.
The effects of temperature of polymerization and type of initiator on stability, incorporation of REACT N1 on latex particles, and wet scrub resistance of paints was also explored. Table 1 also shows the temperature and initiator employed in the polymerizations of Formulations 6 to 8.
The main properties of the emulsions obtained from Formulations 6 to 8 are also presented in Table 1. They had a pH around 8, solid content ranging from 44 to 47 wt%, and particle size in the range of 80 to 90 nm despite using 2 phm of REACT N1. Latexes from Formulations 7 and 8 also presented similar evolution of particle number and level of coagulum in comparison to Formulation 6 (data not shown).
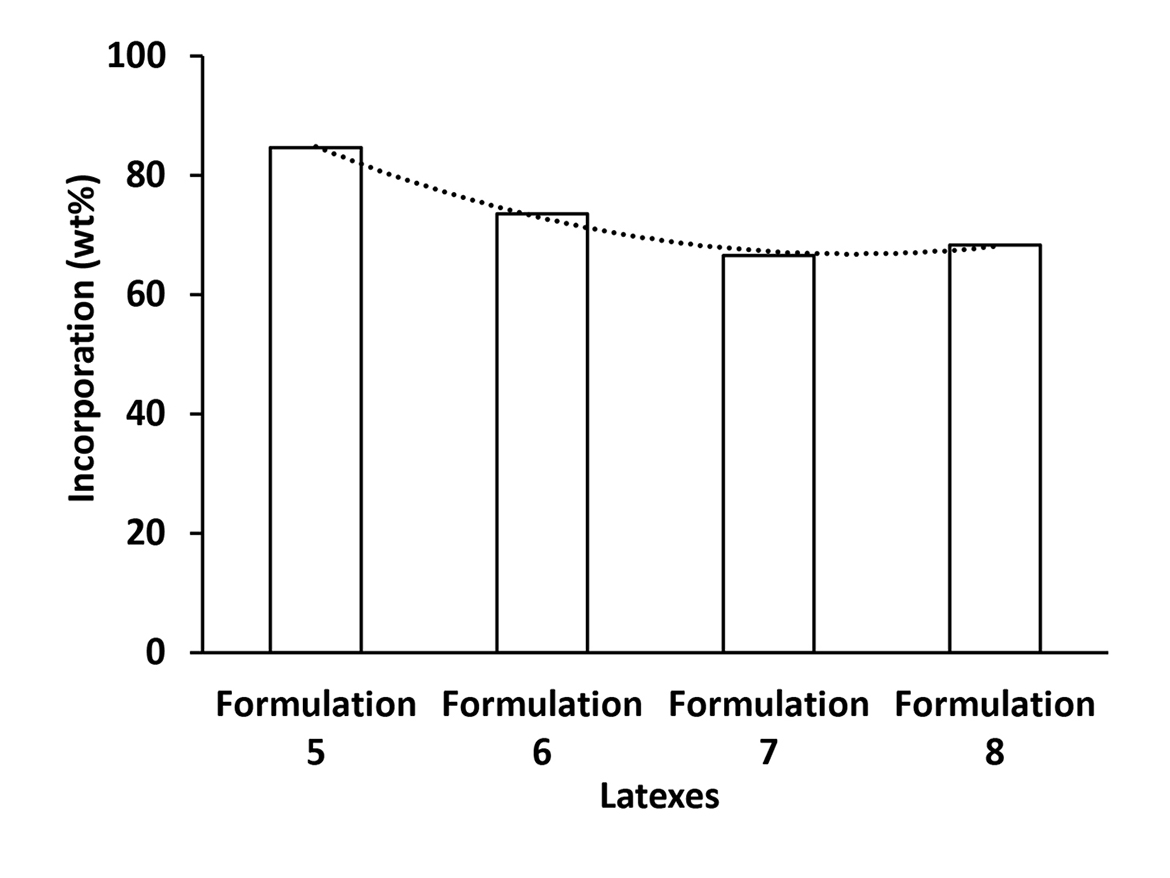
Figure 4 combines all the results of incorporation of REACT N1 into latex particles polymerized according to Process 2. It shows no dependence of the incorporation of REACT N1 into latex particles with the temperature and type of initiator used in the process. However, as mentioned before, it seems to have a dependence of incorporation of REACT N1 into the emulsion particle with the particle coverage since emulsions from Formulations 6 to 8, that received post-addition of conventional anionic surfactant, showed an incorporation around to 70 wt%, while the latex from Formulation 5 without post-addition of conventional anionic surfactant presented an incorporation higher than 80 wt%.
In next Month’s article I will cover the wet scrub resistance and conclusions for this series of experiments.
References
1 Vanderhoff, J. W.; Bradford, E. B.; Carrington, W. K. The Transport of Water through Latex Films, J. Polym. Sci. 1973, 41, 155 - 174.
2 Aramendia, E.; Mallégol, J.; Jeynes, C.; Barandiaran, M. J.; Keddie, J. L.; Asua, J. M. Distribution of Surfactants near Acrylic Latex Film Surfaces: A Comparison of Conventional and Reactive Surfactants (Surfmers), Langmuir 2003, 19, 3212 - 3221.
3 Aramendia, E.; Barandiaran, M. J.; Grade, J.; Blease, T.; Asua, J. M. Improving Water Sensitivity in Acrylic Films Using Surfmers, Langmuir 2005, 21, 1428 - 1435.
4 Noyes, N. Non-Leaching Reactive Surfactants for Architectural Latex Binder, PCI, 2018, 34, 36 - 41.
5 Fithian, P.; O’Shaughnessy, M.; Lubik, M.; Mark, S. Redox for Main Polymerization of Emulsion Polymers, PCI, August2017.
All information contained herein is provided "as is" without any warranties, express or implied, and under no circumstances shall the author or Indorama be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The term "Indorama" is used herein for convenience only, and refers to Indorama Ventures Oxides LLC, its direct and indirect affiliates, and their employees, officers, and directors.
For more information, email the author at Michael.Praw@us.indorama.net.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




