How Much Ionic Strength is Too Much?

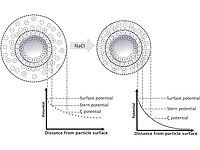
Did you know that we develop guidelines for relating the colloidal stability of latexes to the ionic strength of the aqueous phase via the “critical coagulation concentration,” (CCC)? In our previous “Did You Know?” column, we included a schematic of the ion concentration around latex particles, which allowed us to mention the “diffuse double layer”. It is that halo around the particles that protects the latex from flocculation and eventual coagulation — we insert those images here for reflection.
So the question is, “How much ionic strength is too much?” This is quite interesting as it has been fairly common practice over the years to add some monovalent salts to the latex to reduce its viscosity (i.e. reduce the double layer width). But if too much salt is added — disaster! And remember that total ionic strength includes all ionic species including initiator, buffer, surfactant and adventitious metal impurities that may be in your source water.
This brings us back to the CCC — by definition it is the ionic strength at which there is no net repulsive force between the particles as they approach one another. For monovalent ions, the CCC of many latexes is between 0.1-0.2 molar. But beware of the multi-valent ions. The Schulze-Hardy rule states: CCC is proportional to 1.0/Z6, where Z = the cation valency. For mono-, di- and tri-valent ions, the CCC goes like 1/16 : 1/26 : 1/36, or 1/1 : 1/64 : 1/729 respectively. It then becomes quite obvious why di- and (especially) tri-valent cations are of such concern when we want to have an electrostatically stabilized latex. An extension of this concern and to achieve better stability is the role that non-ionic surfactants can play to mitigate such issues.
This subject is treated in more detail in several of our STEP’n workshops. We welcome comments and further discussion of this topic. Please contact us via our website, www.epced.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!