Ionically Stabilized Latex Viscosity Is Sensitive to the Ionic Strength of the Aqueous Phase

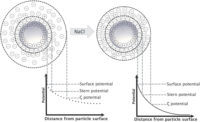
The above statement is true because salt ions affect the thickness of the “double layer” around the particles (see images below). As the double layer shrinks, the latex particles can move around more easily, and the result is a lower viscosity latex. Rheologists call this the “electroviscous effect”. People have used this “trick” for many years to reduce the viscosity in high-solids latexes, but practice dictates that only monovalent ions are useful — divalent ions can destabilize the latex and cause flocculation, if not massive coagulation. It is useful to know that latex particles are VERY close together, even at low solids content. At commercially practical solids levels of 40-50%, the distance between particles is far less than the diameter of the particles (e.g. for 200 nm particles, the interparticle distance is less than 20 nm).
As shown in the image above, a number of well-known scientific/technical terms are identified and defined in terms of the distribution of ions around the latex particles. A commonly measured characteristic of latexes is the Zeta potential (ζ), as seen in the image. It is common to think of the Zeta potential as an indication of the colloidal stability of the latex. Further discussion of these topics is included in our STEP2 workshop on the characterization of latex particles.
As always, we welcome your comments and questions via our website, www.epced.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!