New Elastomeric Binder
With Dirt Pickup Resistance














Developing a coating with improved dirt pickup resistance continues to be an important goal in the coatings industry. Reasons for this include growth in “softer” elastomeric wall and roof coatings; demand for low-VOC formulations, which traditionally result in tackier coatings due to reduced glass transition temperatures (Tgs); and the planned construction of high-rise commercial buildings, most notably in Asia, which is driving the need for coatings that are easier to clean and maintain.
One approach to improving dirt pickup resistance has been to create a harder finish by raising the Tg of the coating. There are, however, downsides to this approach. First, it is nonviable in elastomeric applications, such as elastomeric roof coatings that require elongation and flexibility, since the increased Tg can decrease the flexibility of the coating. Such a decrease in flexibility can lead to the formation of cracks in the coating. Second, increasing the Tg of a coating can require the use of coalescing solvents, which typically have a high VOC content. The increased VOC is in direct opposition to the need to decrease VOCs in coatings due to government regulations.
Other approaches included using highly crosslinked polymers, which try to provide a low-tack surface that impedes dirt penetration. While this method can provide an effective solution for automotive coating applications, architectural and elastomeric coatings provide unique challenges due to the need to retain elongation, making crosslinked polymers a less viable approach.
A New Option
Multi-staged polymers represent fairly new technology that involves a mixture of polymers with different Tg ranges, resulting in a mix of hard and soft segments. This technology, however, has yet to overcome many of the same issues discussed above, specifically elongation properties. The aqueous binder composition of the multi-staged polymers includes (a) polymer particles of average particle diameter with a Tg of -30 °C or lower, and (b) polymer particles of average diameter with a Tg of 50 °C, or higher, where the average diameter ratio between particles of the first polymer and particles of the second polymer must be at least 4:1. To avoid sedimentation, the particle diameter ratio between particles of the first polymer and particles of the second polymer must be no greater than 6:1.
The first and second polymer particles both have a particle size distribution and an average molecular weight that are each in a predetermined value range. The average particle diameter of the first polymer particle ranges from 0.33 to 0.60 micrometers; the average particle diameter of the second polymer particle ranges from 0.06 to 0.09 micrometers. For the various embodiments, the first polymer particle and the second polymer particle each have a high average molecular weight and a polydispersity index (PDI) of no greater than 1.11. Particle size ratio, high molecular weight (MW) and narrow molecular weight distribution are important to assure high dirt pickup resistance.
The combination of the average particle diameter and particle diameter ratio of the first and second polymer particles allows for a percolation threshold volume (Vp) to be obtained when the aqueous coating composition has at least 75 volume percent of the first polymer particle on a dry basis of the aqueous coating composition. It is believed that for the various embodiments, achieving the percolation threshold volume for the particle diameter ratio allows for the second polymer particles (the smaller of the two particles) to preferentially percolate through the first polymer particles to an outer surface of the elastomeric coating where they can help to form a hard and rough skin layer that improves dirt pickup resistance.
The first polymer particle and the second polymer particle each include a hydrophobic branched monomer in polymerized form; particles were synthesized by a free radical polymerization process prepared with a hydrophobic branched monomer. For the various embodiments, the hydrophobic branched monomer in the first polymer particle is a NEO monomer with a Tg of -40 °C. For the second polymer particle the hydrophobic branched monomer is a NEO monomer with Tg of +70 °C.
The aqueous binder composition does not require additional components, such as solvents and/or coalescents to form a film. For the various embodiments, the elastomeric binder formed can provide an elongation value of 850-1000%, determined according to ASTM D2370. Likewise, the elastomeric binder gives the possibility of formulating elastomeric wall paints with a vapor transmission of 5 to 9 g/m2/day determined according to ASTM F1249 or TAPPI 448, and a water absorption (after 96 h soaking in tap water) of 9.0% or less. For the various embodiments, the elastomeric coating formed with the aqueous coating composition can provide a contact angle of at least 128° determined according to ASTM D7334.
The binder synthesized according to this technology also shows high dirt pickup resistance, which is a consequence of several technical facts such as the high reactivity of the branched hydrophobic monomers used (which gives a high-MW polymer with low polydispersity), the ratio of particle size between the two binders, the low polydispersity of each polymer to be blended, the relative binder composition, and the usage of a UV-reactive monomer to synthesize the low-particle-size hard polymer. These properties allow the formulation of elastomeric coatings that are particularly well suited for use on masonry, concrete surfaces and stone surfaces, among others.
As has been mentioned, the first and the second polymer in the aqueous binder composition have an average particle size distribution that is very narrow. In other words, both polymers to be blended have very small polydispersity (standard deviation of the average particle size distribution). For example, the polydispersity for the first polymer particle can be 5% or less, while the polydispersity for the second polymer particle can be 7% or less. As a result, the aqueous coating composition can have essentially a bimodal particle size distribution, or a binary mixture, of the first and second polymer particles.
The bimodal distribution and the particle diameter ratio of the first and second polymer particles have an influence on how the polymer particles segregate during the formation of the elastomeric coating. As appreciated, a system of particles in motion (such as the first and second polymer particles in the aqueous coating composition as the elastomeric coating is forming) distributes itself through a variety of mechanisms, including what is known as percolation. During percolation, different-sized particles of the system can migrate in different directions depending upon a number of different factors. These factors can include the relative size and weight of the particles as well as the temperature at which the percolation is occurring. As a result of this migration, the different-sized particles can segregate themselves to different parts of the elastomeric coating.
A Hard and Rough Layer
For the various embodiments, the particle diameter ratio (with its bimodal distribution) and the weight average molecular weight of the first and second polymer particles, among other things, are believed to affect the segregation of the polymer particles as the elastomeric coating forms. In particular, a Vp has been identified from these parameters that provides a volume percentage of the second polymer particle (the relatively smaller hard polymer particle as compared to the first polymer particle) needed to cause the second polymer particles to preferentially segregate to an outer surface of the elastomeric coating during the drying process. In this relative position, the second polymer particles can help to form a hard and rough layer that is hydrophobic and that helps to improve dirt pickup resistance, while the first polymer particle helps to balance and control the elastomeric behavior of the elastomeric coating. For the various embodiments, the Vp can be obtained with aqueous coating compositions having at least 75 volume percent of the first polymer particle on a dry basis of the aqueous coating composition; the remaining volume percent of the aqueous coating composition can be the second polymer particle.
There is not necessarily a complete segregation of the first and second polymer particles as the elastomeric coating forms. For the various embodiments, the hard and rough layer of the elastomeric coating can include a blend of the first and second polymer particles. Such blends, however, will typically include a majority of the second polymer particle when the volume percentage of the second polymer particle is within the Vp. In other words, the Vp of the present disclosure can be used to better ensure that the bimodal system of the first and second polymer particles will preferentially segregate so that the majority of the hard and rough layer is formed with the second polymer particles. It mostly depends on the particle size polydispersity.
Even more surprisingly, it has also been found that when the volume percentage of the second polymer particle is within the percolation threshold volume, the aqueous coating composition does not require a coalescing agent in order to form the elastomeric coating.
For the various embodiments, the morphological structure of the hard and rough layer also contributes to the elastomeric coating’s ability to provide dirt pickup resistance. As illustrated in the following sections, the hard and rough layer of the elastomeric coating includes a topography having projections or bumps that provide for a rough surface. One skilled in the art will appreciate that the presence of a relatively high degree of surface roughness can provide for at least two important contact effects between the rough surface and materials that can come into contact with the rough surface. First, the existence of a high degree of surface roughness can provide for a very small contact area between the surface and a contaminant (e.g., a particulate or an aqueous liquid droplet) that can come into contact with the surface. As such, adhesion between the contaminant and the surface can be minimized due to the minimal contact area between the two. Second, the surface roughness can facilitate the trapping of air beneath a portion of the contaminant. For instance, when considering a liquid droplet coming into contact with the rough surface, an air boundary layer can form between portions of the droplet and the surface; this air boundary layer can increase the contact angle between the droplet and the surface.
Although surface roughness can provide a surface with some degree of hydrophobicity, hydrophobicity can be further enhanced when combined with a surface chemistry providing a low surface energy. The hard and rough layer of the elastomeric coating also displays a low surface energy, which, coupled with the rough surface, leads to a high contact angle that resists wetting and adherence of dirt and contaminants. Thus, when a solid particulate or a liquid droplet, (e.g., a water droplet) contacts the coating, it can roll down or slide off of the surface due to the combined effects of surface roughness and low surface energy. Also, when considering a liquid droplet, as the droplet rolls down the surface and encounters a solid particle on the surface, the particle can adhere to the passing droplet and can simultaneously be removed from the surface with the liquid, as adhesion between the surface and the particle has been minimized. Thus, the particle can preferentially adhere to the liquid and be “cleaned” from the surface of the elastomeric coating.
It has been discovered that if a coalescing agent is used, the resulting hard and rough layer structure is altered to a smoother surface relative to the hard and rough surface formed without the use of the coalescing agent. Therefore, the use of coalescent aids is not recommended.
Forming the Polymer Particles
For the various embodiments, elastomeric coatings formed with the aqueous coating composition of the present disclosure can have contact angles of 128° minimum.
Known synthesis techniques have been used to control the polymer particle size. Surfactant type and the polymerization process, among other things, have a great influence on the polymer particle size. The size and polydispersity of the polymer particles have been controlled by the choice of polymerization starting materials and conditions for the first and second polymer, such as seed size and concentration, polymerization rate, catalyst or initiator concentration, reaction temperature, surfactant concentration, etc.
The first polymer and the second polymer can each be prepared by an emulsion polymerization process using at least one hydrophobic ethylenically unsaturated monomer and several hydrophilic ones.
These hydrophilic functional monomers that are useful in forming the first and second polymer particles can include, but are not limited to, hydrophilic functional monomers that contain ethylenically unsaturated double bonds for free radical reaction with the hydrophobic ethylenically unsaturated monomer or other monomers during polymerization. Examples of such hydrophilic functional monomers can include acrylic acid, methacrylic acid, n-butyl acrylate, 2-ethylhexyl acrylate, methyl methacrylate, acrylamide, and mixtures thereof.
Monomers used in forming the first polymer particle can include mixtures of 2-ethylhexyl acrylate and/or n-butyl acrylate, NEO monomer with Tg of -40 °C, acrylic or methacrylic acid, and/or acrylamide, and methyl methacrylate that contains more than 40% by weight of NEO monomer. The remaining monomer is mostly 2-ethylhexyl acrylate, n-butyl acrylate or combination thereof.
Monomers used in forming the second polymer particle can include mixtures of NEO monomer with Tg of +70 °C and several hydrophilic ones such as 2-ethylhexyl acrylate and/or butyl acrylate, acrylic or methacrylic acid, acrylamide and methyl methacrylate that contains more than 40% by weight of NEO monomer, no more than 5% by weight of methyl methacrylate, and less than 3% of 4-(2´-acryloyloxyethoxy)-2-hydroxybenzophenone, with the remaining monomer being 2-ethylhexyl acrylate, n-butyl acrylate or a combination thereof.
Suitable polymerization conditions must be used. Typically, the reaction temperature is 70-80 °C. The polymerization can be conducted using polymerization initiators. Suitable free radical polymerization initiators are known to promote emulsion polymerization and can include water-soluble oxidizing agents, such as organic peroxides (e.g., t-butyl hydroperoxide, cumene hydroperoxide, etc.), inorganic oxidizing agents (e.g., hydrogen peroxide, potassium persulfate, sodium persulfate, ammonium persulfate, etc.), and those initiators that are activated in the water phase by a water-soluble reducing agent. Such initiators are employed in an amount sufficient to cause polymerization. The amount of such free radical initiators used can be in the range of 0.05-0.20% by weight based on the weight of all monomers present.
Redox initiators may be employed, especially when polymerization is carried out at lower temperatures. For example, reducing agents may be used in addition to the persulfate and peroxide initiators mentioned above. Typical reducing agents can include, but are not limited to, alkali metal salts of hydrosulfites, sulfoxylates, thiosulfates, sulfites, bisulfites, etc. In general, the reducing agents are used in the range of 0.01-0.20% by weight based on the weight of all monomers present.
Various additives can be added before, during or after polymerization. These include surfactants, reactive surfactants, radical chelating agents, buffering agents, neutralizing agents, defoamers and polymeric stabilizers, among others. Suitable surfactants can include, but are not limited to, those having a low critical micelle concentration (CMC). For the various embodiments, suitable surfactants have a CMC of less than 0.009 g/100 g in 0.1 M NaCl at 25 °C. So, for the various embodiments the first polymer and the second polymer include surfactant having a critical micelle concentration of less than 0.009 g/100 g in 0.1 M NaCl at 25 °C combined with reactive surfactants.
Examples of suitable surfactants can include alkyldiphenyloxide disulfonate, polyoxyethylene tridecyl ether phosphate, ammonium salt, and polyoxyethylene alkylphenyl ether ammonium sulfate. For the various embodiments, the amount of the surfactant can be in the range of 0-2.8% by weight.
Study 1: Percolation Threshold
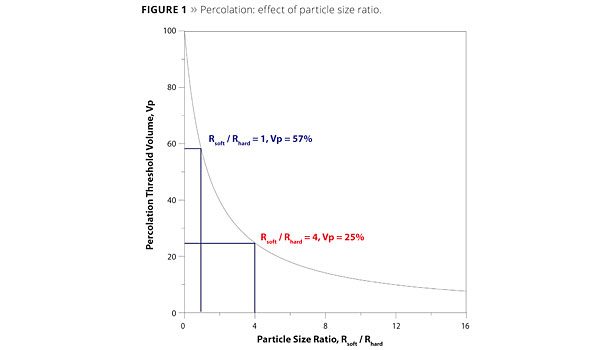
In this example, the percolation threshold volume, Vp, as a function of the particle diameter ratio was studied. As discussed earlier, the Vp provides a volume percentage of the second polymer particle (the relatively smaller, hard polymer particle as compared to the first polymer particle) needed to cause the second polymer particles to preferentially segregate to an outer surface of the elastomeric coating during the drying process. The bimodal distribution and the particle diameter ratio of the first and second polymer particles have an influence on how the polymer particles segregate during the formation of the elastomeric polymer film. As shown in Figure 1, as the particle diameter ratio increased, the Vp decreased. As a result, a lower concentration of the second polymer particle (hard) is required to achieve the Vp. For the various embodiments, improvements in the dirt pickup resistance and other properties of the elastomeric coating are also observed as the particle diameter ratio increases. Values for the particle diameter ratio that provide for the Vp, as well as dirt pickup resistance and elongation properties, include those where the first average particle diameter to the second average particle diameter have a particle diameter ratio in the range of 4:1 to 6:1. Particle diameter ratio values above 6:1 have been found to cause sedimentation of the first polymer particle in the aqueous coating composition over time.
From the data provided in Figure 1, aqueous coating compositions of the first and second polymer particles at percolation threshold volumes of 57% and 25% were prepared. For the aqueous coating compositions with the percolation threshold volumes of 57%, the first polymer particle had a PDI of 1.09, a Tg of -40 °C, high MW and an average particle diameter of 0.1 micron. The second polymer particle had a PDI of 1.08, a Tg of 50 °C, high MW and an average particle diameter of 0.09 micron. The volume average particle diameters for the first and second polymer particles allow for aqueous coating compositions having particle diameter ratios of 1.11:1 to achieve the Vp of 57%. In this case, due to the volume of hard polymer necessary to achieve percolation, a non-elastomeric behavior is expected. The volume average particle diameters of the first and second particles are determined based on spherical geometry using diameter measurements from a Nanotrac®150 (Microtrac, Inc.) dynamic light scattering device, where the measurements were taken on a 1 weight percent aqueous suspension of the particles in distilled water.
For the aqueous coating compositions with the percolation threshold volumes of 25%, the first polymer particle had a PDI of 1.08, a Tg of -40 °C, high MW and an average particle diameter of 0.36 micron. The second polymer particle had a PDI of 1.08, a Tg of 70 °C, high MW and an average particle diameter of 0.09 micron. The volume average particle diameters for the first and second polymer particles allow for aqueous coating compositions having particle diameter ratios of 4:1 to achieve the Vp of 25%. An elastomeric behavior was observed. Here the average particle diameter of the first and second particles were also determined based on spherical geometry using diameter measurements from a Nanotrac 150.
For each of the aqueous coating compositions, 75 volume percent of the first polymer particle and 25 volume percent of the second polymer particle were mixed on a dry basis of the aqueous coating composition in a Heidolph ST1 agitator at 200 RPM for 15 min at 25 °C. The composition was allowed to rest 24 h before preparing the elastomeric coating. An elastomeric coating was formed with each of the aqueous coating compositions by applying a 0.007 inch (7 mils)-thick coat of the aqueous coating composition to a Leneta P121-10N chart (Leneta Co.) using a Dow Latex Applicator U from BYK-Gardner. The aqueous coating composition was allowed to dry at 25 °C and a controlled relative humidity of 50% for 24 h to form the elastomeric coating. Each of the elastomeric coatings was sputter coated and viewed under a Hitachi S2400 scanning electron microscope (Hitachi Instruments Inc.).
Figure 2 provides an SEM image of the elastomeric coating formed with the aqueous coating composition described above having the particle diameter ratio of 1.11:1 and the percolation threshold of 57%. As shown, the second polymer particles (the hard polymer) are present at the skin layer in about the same proportion as in the polymer particles supporting the skin layer, in direct proportion to their concentration. This would be expected from random packing of compatible latexes having similar particle sizes (particle diameter ratio almost equal to 1). Percolation according to the present disclosure was not seen in this aqueous coating composition. For this case, the only way to achieve percolation was to prepare a blend with 57% in volume of hard polymer, which gives a non-elastomeric binder. In contrast, Figure 3 provides an SEM image of an elastomeric coating formed with the aqueous coating composition having a particle diameter ratio of 4:1 and a percolation threshold of 25%. As shown, the second polymer particles have preferentially segregated to the outer surface of the elastomeric coating during the drying process. In this relative position, the second polymer particles can help to form the hard and rough layer of the elastomeric coating that is both hydrophobic and that helps to improve dirt pickup resistance, while the first polymer particle helps to balance and control the elastomeric behavior of the elastomeric coating.
Study 2: Bending Resistance
In this example, bending resistance of the elastomeric coatings as a function of the Tg values for the first polymer particle and the second polymer particle used in the aqueous binder compositions was studied. In particular, the Tg of the first polymer particle was varied, while the Tg of the second polymer particle was kept constant.
The aqueous coating composition of the first and second polymer particles at a percolation threshold volume of 25%, which corresponds to a particle diameter ratio of 4:1, was prepared. For this example, the first polymer particle had a PDI of 1.08, high MW, an average particle diameter of 0.36 and Tg values starting with -10 °C and following with -20, -30 and -40 °C. The second polymer particle had a PDI of 1.08, high MW, a volume average particle diameter of 0.09 microns and a Tg value of 50 °C. The Tg values for the first and second polymer particles were determined by differential scanning calorimetry using a DSC Q 1000 from TA Instruments.
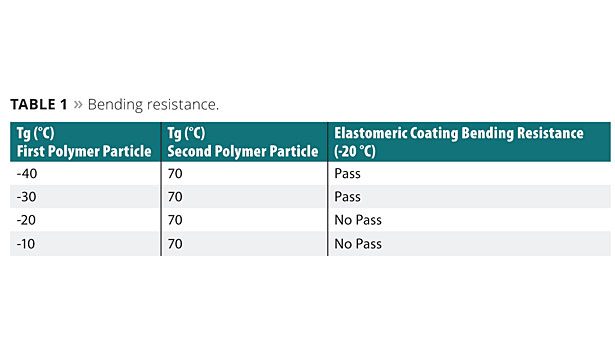
Each of the aqueous coating compositions was formed in the same manner as in Example 1. An elastomeric coating was formed with each of the aqueous coating compositions by forming a 1.2 millimeter-thick coating of the aqueous coating composition on a glass plate. The aqueous coating composition was allowed to dry at 25 °C at a controlled relative humidity of 50% for seven days to form the elastomeric coatings. The coating was removed from the glass plate, and the bending resistance of each coating was tested according to ASTM D522 Standard Test Methods for Mandrel Bend Test using an Elcometer 1510 conical mandrel bend tester. For results in Table 1, a “Pass” means that no cracks were seen in the elastomeric coating after the bending resistance test. “No Pass” means that cracks were seen in the elastomeric coating after the bending resistance test.
Table 1 shows that as the Tg of the first polymer particle increases, the bending resistance of the elastomeric coating does not pass.
Study 3: Tack and Film Crack Properties
In this example, the residual tack and film crack properties of the elastomeric coatings as a function of the Tg values for the first polymer particle and the second polymer particle used in the aqueous coating compositions were evaluated. In particular, the Tg of the first polymer particle was kept constant while the Tg of the second polymer particle was varied.
The aqueous coating composition of the first and second polymer particles at a percolation threshold volume of 25%, which corresponds to a particle diameter ratio of 4:1, was prepared. For this example, the first polymer particle had a PDI of 1.08, high MW, a volume average particle diameter of 0.36 and a Tg value of -30 °C. The second polymer particle had a PDI of 1.08, high MW, a volume average particle diameter of 0.09 and Tg values of 40, 45, 70 and 90 °C. The Tg values for the first and second polymer particles were determined by differential scanning calorimetry.
Each of the aqueous coating compositions was formed in the same manner as the previous studies. An elastomeric coating was formed with each of the aqueous coating compositions by applying a 0.007 inch-thick coat of the aqueous coating composition to a Leneta P121-10N chart. The aqueous coating composition was allowed to dry at 25 °C and a controlled relative humidity of 50% for 24 h to form the elastomeric coating.
The residual tack of each coating was tested using a Rolling Ball test method according to ASTM D3121. For the test of residual tack, the distance in centimeters the ball rolls determines the presence of residual tack, where distances less than 20 centimeters indicate a coating with an unacceptable tack, and distances of 20 centimeters or greater indicate a coating with an acceptable tack or no tack. A TT-100 Rolling Ball Track Tester, which meets standards set by the Pressure Sensitive Tape Council (PSTC-6) and the ASTM (ASTM D3121) was used for testing tack of the film.
The film crack properties of each of the elastomeric coatings were also tested and the presence of cracks determined by visual observation according to ASTM D823.
The results for both the residual tack and the film crack properties are shown in Table 2.
As shown in Table 2, as the Tg of the second polymer particle is increased, the elastomeric coating transitions from having a residual tack (Tg of 40 °C for the second polymer particle) to not having a residual tack at Tg values of 70 °C and greater. However, if the Tg of the second polymer particle is increased to a value of 90 °C or above, the elastomeric coating shows film crack.
Study 4: Soiling Test and Elongation
In this example, a soiling test was used to measure dirt pickup resistance of elastomeric coatings formed with aqueous binder compositions having different particle diameter ratios. In addition, elongation of the elastomeric coatings formed with aqueous binder compositions having different particle diameter ratios was measured.
Aqueous binder compositions of the first and second polymer particles having particle diameter ratios of 3:1, 4:1 and 6:1 were prepared. For this study, the first polymer particle had a PDI of 1.08, a Tg of -30 °C, high MW, and average particle diameters of either 0.27, 0.36 or 0.54. The second polymer particle had a PDI of 1.08, a Tg of 70 °C, high MW and an average particle diameter of 0.09. The average particle diameters for the first and second polymer particles allow for aqueous binder compositions having particle diameter ratios of 3:1, 4:1 and 6:1 to achieve the Vp of 28%, 25% and 16%, respectively. The average particle diameters of the first and second particles were determined based on spherical geometry using diameter measurements from a Nanotrac 150 device, where the measurements were taken on a 1 weight percent aqueous suspension of the particles in distilled water.
Each of the aqueous coating compositions was formed in the same manner as in the previous examples. Allow the composition to rest 24 hours before preparing the elastomeric coating.
For the soiling test, an elastomeric coating was formed with each of the aqueous coating compositions by applying a 0.007 inch (7 mils)-thick coat of the aqueous coating composition to the varnished side of a Leneta Form 2C opacity chart. The aqueous coating composition was allowed to dry at 25 °C and a controlled relative humidity of 50% for 24 h to form the elastomeric coating.
An initial reflectance of each coating was measured and recorded using a Technibrite™ Micro TB-1C (measurement angle of 45° using a 457 nm wavelength). The entire surface of the elastomeric coating was soiled with a slurry of iron oxide in water (50% weight/weight slurry of red iron oxide and water). To soil the elastomeric coating, the slurry was applied using a brush at 25 °C. The soiled coating was placed in an oven (Blue M Industrial Oven) set to 60 °C for 8 h. The coating was allowed to cool to room temperature and was then washed with high-pressure water (tap water at room temperature) using an air pistol (Cane NT) working at a pressure of 40 PSI and at a distance of 20 to 30 cm from the soiled coating. The coating was then dried at room temperature and humidity. This soiling process was repeated a total of five times.
After the fifth soiling process, a final reflectance of the soiled coating was measured using the Technibrite at the same setting used to measure the initial reflectance. The initial reflectance of the elastomeric coating and the final reflectance of the soiled coating were used to calculate a percent drop of reflectance using the following equation:
% Drop of Reflectance = [(Initial reflectance) – (Final Reflectance) / Initial Reflectance] x 100
To test elongation of the elastomeric coatings, an elastomeric coating was formed with each of the aqueous coating compositions by forming a 1.2 mm-thick coating of the aqueous coating composition on a glass plate. The coating was allowed to dry at 25 °C and a controlled relative humidity of 50% for seven days to form the elastomeric coating. The coating was removed from the glass plate, and the elongation of the elastomeric coating was measured according to ASTM D2370 using an Instron 1011.
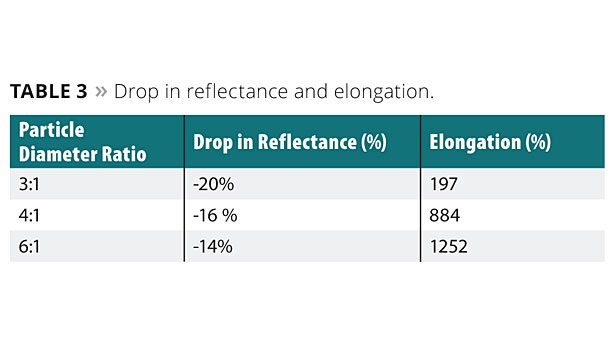
Table 3 provides data on the percent drop in reflectance for the soiled elastomeric coatings, where the larger the percent drop in reflectance the lower the dirt pickup resistance. Table 3 also provides data on the elongation of the elastomeric coatings.
As can be seen, the aqueous coating composition having a particle diameter ratio of 6:1 provided a percent drop in reflectance and an elongation that is superior to the aqueous coating composition having a particle diameter ratio of 3:1. As discussed earlier, for the given Tgs, the particle diameter ratio has an impact on the efficiency of the percolation of the aqueous coating composition. It is believed that particle diameter ratios of less than 4:1 for the aqueous coating composition percolate less efficiently, which results in less of the second polymer particle in the skin layer of the elastomeric coating as compared to ratios of 4:1 or 6:1. This less-efficient percolation results in more of the second polymer particle remaining below the skin layer of the elastomeric coating, where it causes an increase in the overall Tg of the elastomeric coating below the skin layer. In addition, when more of the second polymer particle is present below the skin layer, it may form hard polymer domains that create discontinuities in the elastomeric coating below the skin layer, leading to the negative effect on the elongation of the elastomeric coating as illustrated in Table 3.
Study 5: Blistering Resistance, Elongation, Dirt Pickup Resistance and Porosity
In this example, the aqueous coating composition was used as a binder in paint formulations with different pigment volume concentrations (PVCs). Elastomeric coatings formed with the paint formulations were tested for blistering resistance, elongation, dirt pickup resistance and porosity.
The aqueous coating composition of the first and second polymer particles at a Vp of 25%, which corresponds to a particle diameter ratio of 4:1, was prepared. For this example, the first polymer particle had a PDI of 1.08, high MW, a volume average particle diameter of 0.36 and a Tg value of -30 °C. The second polymer particle had a PDI of 1.08, high MW, a volume average particle diameter of 0.09 microns and a Tg value of 70 °C. Each of the aqueous coating compositions was formed in the same manner as in earlier studies. The aqueous binder composition had 60 weight % solids content.
The paint was formulated in two steps. In the first step, a grind was prepared by adding to a 1 Kg glass beaker 44.00 weight % water, 0.12 weight % defoamer, 0.40 weight % dispersing agent, 0.10 weight % non-ionic surfactant and 0.40 weight % HEC ER-30,000 thickener, while mixing at low speed (no more than 20 RPM) with a COWLES mixer (Morehouse Cowles) for 10 min at room temperature.
To prepare the grind, 20.00 weight % hydrophobic-modified titanium dioxide pigment, 16.90 weight % high-purity silica produced from synthesized cristobalite extender and 17.97 weight % hydrous aluminum silicate treated extender were stirred in at high speed (more than 50 RPM). After adding the last extender, agitation was maintained at high speed for 1 h. Finally, 0.046 weight % OIT (fungicide) and 0.064 weight % isothiazolinone blend 14% were added as bactericides.
In the second step, the letdown was prepared at a speed of no more than 20 RPM by adding the aqueous coating composition (used as the binder) to the grind to obtain three different PVCs of 20%, 42% and 55%. For a PVC of 20%, 40 weight % grind was blended with 60 weight % aqueous coating composition. For a PVC of 42%, 66 weight % grind was blended with 34 weight % aqueous coating composition. For a PVC of 55%, 76 weight % grind was blended with 24 weight % aqueous coating composition. The resulting paint formulations were mixed an additional 10 min at 10 RPM. The pH was adjusted to 8 using ammonia or other base.
To test blistering resistance, a coating was formed with each of the paint formulations by forming a 1.2 mm-thick coating of the aqueous coating composition on a glass plate. The aqueous coating composition was allowed to dry at 25 °C and controlled relative humidity of 50% for seven days to form the elastomeric coating. The coating was removed from the glass plate and was cut into a 5 cm by 5 cm test specimen. The specimen was placed in a glass beaker filled with tap water and allowed to soak at room temperature for 96 h. The specimen was removed from the tap water, and the surface was dried with a paper tissue. The blister resistance was tested according to ASTM D714.
To test elongation, a coating was formed with each of the paint formulations as discussed above for the blistering resistance test. The elastomeric coating was removed from the glass plate, and the elongation of the elastomeric coatings prepared with the different paint formulations was measured according to ASTM D2370 using an Instron 1011.
Drop of reflectance for the elastomeric coatings prepared with the paint formulations using the soiling test discussed above in Study 4 was also measured.
To test the porosity of the elastomeric coatings formed from the paint formulations, a coating was formed with each of the paint formulations by applying a 0.007 inch (7 mils)-thick coat of the paint formulation to a Leneta P121-10N chart. The paint formulation was allowed to dry at 25 °C and controlled relative humidity of 50% for 24 h to form the elastomeric coating. The porosity of the elastomeric coatings prepared from the paint formulations was tested according to ASTM D3258.
Table 4 shows the results for blister resistance, elongation, dirt pickup resistance (as measured by drop in reflectance), and porosity for coatings prepared with the paint formulations.
As shown in Table 4, elastomeric coatings prepared from the paint formulation having a PVC of 20% have high elongation, very low porosity resulting in poor blister resistance and a 17% drop in reflectance after the soiling test. Elastomeric coatings prepared from the 42% PVC have a lower elongation as compared to the elastomeric coating prepared from the paint formulation having a PVC of 20%, a 17% drop in reflectance after the soiling test, very low porosity, but good blister resistance. It is believed that PVC of 42% is very close to the critical PVC for the paint formulation as prepared for this study
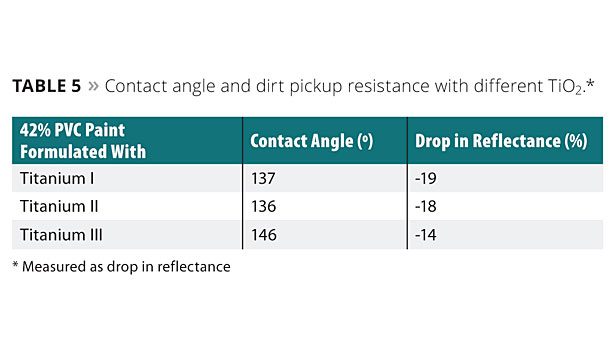
Study 6: Contact Angle and Dirt Pickup Resistance with Different TiO2
In this example, three paint formulations having a 42% PVC, with different types of titanium dioxide, were prepared. The elastomeric coatings formed from the paint formulations were tested for contact angle and dirt pickup resistance. The different types of titanium dioxide for use in the paint formulations were a multipurpose rutile titanium dioxide pigment manufactured by the chloride process with 93% TiO2 (Titanium I), a rutile titanium dioxide pigment designed to provide maximum hiding in high-quality, high-PVC architectural coatings with 80% TiO2 (Titanium II), and a universal rutile titanium dioxide pigment, manufactured by the chloride process, that is designed to deliver both high gloss and excellent durability in coatings with 93% TiO2 (Titanium III). Each type of titanium dioxide had surface treatment with amorphous silica and alumina at different weight percentages. Of the three titanium dioxides, Titanium III had a hydrophobic surface treatment.
To test the contact angle, a coating was formed in the same manner as above. Contact angles were measured using a Dataphysics OCA 150 equipment according to ASTM D7334 (Pendant drop method).
To test drop in reflectance, a drawdown of each paint formulation was prepared by applying a 0.007 inch-thick coat of the paint formulation to the varnished side of a Leneta Form 2C opacity chart. Drop of reflectance was measured using the soiling test discussed in Example 4.
Table 5 shows the results for the contact angle and the drop of reflectance for the elastomeric coatings prepared with the 42% PVC paint formulations having different titanium dioxide.
As shown in Table 5, Titanium III gives the paint formulation the lowest drop in reflectance and the highest contact angle as compared to the other types of titanium dioxides tested.
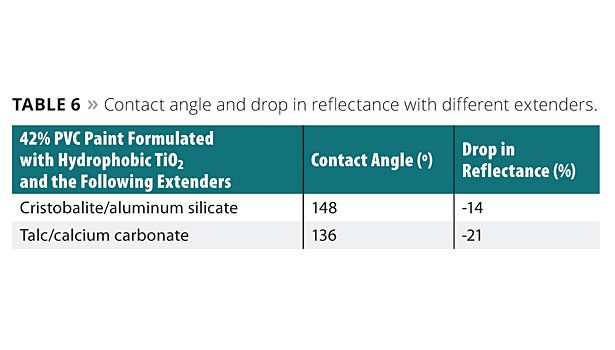
Study 7: Contact Angle and Dirt Pickup Resistance with Different Extenders
In this example, two paint formulations having a 42% PVC, with different combinations of extenders were prepared. The elastomeric coatings formed from the paint formulations were evaluated for contact angle and dirt pickup resistance. The different types of extenders included high-purity silica produced from synthesized cristobalite extender, a hydrous aluminum silicate, treated extender, platy talc and calcium carbonate. Cristobalite and aluminum silicate have a hydrophobic behavior. Platy talc is less hydrophobic than cristobalite and calcium carbonate is a hydrophilic calcium carbonate.
The paints were formulated as described in Example 5, where the first paint formulation included the following combination of extenders: cristobalite and aluminum silicate; a second paint formulation included the following combination of extenders: talc and calcium carbonate. For the second combination, the paint formulation included 16.90 weight % talc and 17.97 weight % calcium carbonate
To test drop in reflectance, a coating was formed with each of the paint formulations by applying a 0.007 inch (7 mils)-thick coat of the paint formulation to the varnished side of a Leneta Form 2C opacity chart. The paint formulation was allowed to dry at 25 °C and a controlled relative humidity of 50% for 24 h to form the elastomeric coating. Drop of reflectance for the elastomeric coatings prepared with the paint formulations was measured using the soiling test.
To test the contact angle, a coating was formed with each of the paint formulations by applying a 0.007 inch-thick coat of the aqueous coating composition to a Leneta P121-10N chart. The paint formulation was allowed to dry at 25 °C and controlled relative humidity of 50% for 24 h to form the elastomeric coating. Contact angles were measured using a Dataphysics OCA 150 according to ASTM D7334 (Pendant drop method).
Table 6 shows the results for the contact angle and the drop of reflectance for the elastomeric coatings prepared with the 42% PVC paint formulations using different combinations of extenders.
As shown in Table 6, the use of the first combination of extenders (cristobalite and aluminum silicate) provided for a more hydrophobic coating as compared to the second combination of extenders (talc and calcium carbonate). The lower drop in reflectance showed by the paint formulated with the first combination of extenders also confirms the higher dirt pickup resistance typically seen on rough, hydrophobic surfaces.
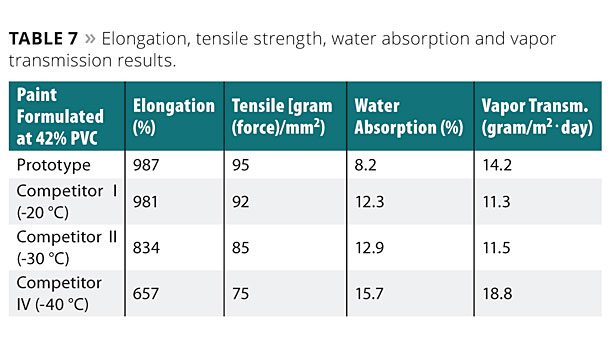
Study 8: Elongation, Tensile Strength, Water Absorption and Vapor Transmission
In this example, four paint formulations, each having a 42% PVC, were prepared. The first paint formulation used the aqueous binder composition (prototype) as the binder (prepared as described in Example 5). The second example was the same paint formulation using a competitive polymer (Tg of -20 °C) as a binder. The third paint formulation used another competitor as the binder (Tg of -30 °C), and the fourth paint formulation used a commercial polymer with Tg of - 40 °C as a binder. The coatings formed with the paint formulations were tested for elongation, tensile strength, water absorption and vapor transmission.
To test elongation, tensile strength, water absorption and vapor transmission of the coatings, a 1.2 mm film with each of the paint formulations was prepared. The formulations were allowed to dry at 25 °C and controlled relative humidity of 50% for seven days to form the coating. For the elongation and tensile strength, the coating was removed from the glass plate, and the elongation and tensile strength were measured according to ASTM D2370 using an Instron 1011. Water absorption was measured, as discussed herein, and vapor transmission according to ASTM F1249. The results are shown in Table 7.
Study 9: Soiling Test
In this example, four paint formulations were prepared. The first one had a 42% PVC using the same paint formulation described in Example 5 and the prototype with ratio of particle size 4:1 and 75%/25% blend as a binder. The second paint formulation had a 42% PVC using the same paint formulation described in Example 5 with the prototype binder with particle size 4:1, but with the composition 81%/19% as a binder. The third paint formulation had a 42% PVC using the same paint formulation described in Example 5, where Competitor II was the binder. The fourth paint formulation had 42% PVC using the same paint formulation described in Example 5, where Competitor III was the binder.
For the soiling test, drawdowns of each paint formulation were made by applying a 0.007 inch-thick coat to the varnished side of a Leneta Form 2C opacity chart. The paint formulation was allowed to dry at 25 °C and a controlled relative humidity of 50% for 24 h.
Initial reflectance of each coating was measured using a Technibrite (measurement angle of 45° using a 457 nm wavelength). Then the entire surface of the coating was soiled with a slurry of iron oxide in water (50% weight/weight slurry of red iron oxide and water) using a brush at 25 °C. The soiled coating was placed in an oven set to 60 °C for 8 h. The soiled coating was allowed to cool to room temperature. Finally the coating was washed with high-pressure water (tap water at room temperature) using an air pistol (Cane NT) working at a pressure of 40 PSI and at a distance of 20 to 30 cm from the soiled coating. The coating was allowed to dry at room temperature and humidity. This soiling process was repeated a total of five times.
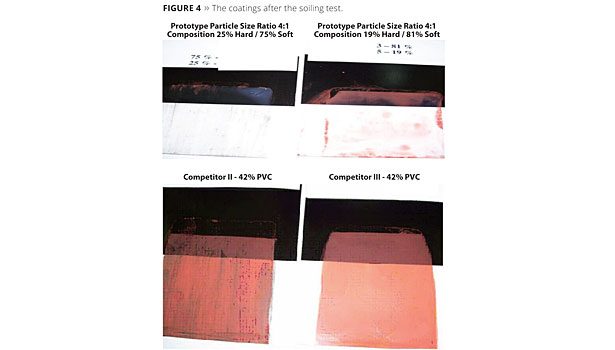
Figure 4 provides images of the coatings after the soiling test, where the reflectance is measured in a region of the soiled coatings that have been washed as discussed above. For the images, the dark portions in the region indicate the presence of soil on the coatings. As can be seen, a visual comparative evaluation demonstrates that the elastomeric coatings formulated with the aqueous coating composition as the binder (the first and second) provides superior dirt pickup resistance as compared to the other two coatings (the third and fourth paint formulations).
It is also easy to see that the second paint formulation shows poorer performance than the first one. It is justified by the fact that for this example the amount of hard polymer present is lower than the one necessary to achieve percolation for the particle size ratio 4:1
Study 10: Contact Angle
In this example, a paint formulation having 42% PVC, using the prototype with particle size ratio 4:1 and with composition of 75% soft polymer and 25% hard polymer, was prepared. A drawdown was made by applying a 0.007 inch-thick coat of the paint formulation to a Lenexa P121-10N chart. The paint formulation was allowed to dry at 25 °C and controlled relative humidity of 50% for 24 h. Contact angle was measured using Data physics OCA 150 according to ASTM D7334 (Pendant drop method).
The elastomeric coating produces a contact angle of 142°. Figure 5 shows a picture of a drop of water on the elastomeric coating, whose shape indicates that the surface of the elastomeric coating is hydrophobic.
Summary
This new binder, adequately formulated, gives coalescent-free elastomeric wall paints and elastomeric roof coatings with elongation values of 500 to 1,000% (according to ASTM D2370), high storage modulus, which means good elongation retention within a wide range of temperatures, a vapor transmission of 5 to 9 g/m2/day determined according to ASTM F 1249, a water absorption lower than 9.0% after soaking in tap water for 96 h, a minimum contact angle of 138° and excellent dirt pickup resistance.
References
1. Kusy, R.P. Influence of Particle Size Ratio on the Continuity of Aggregates, J. Appl. Phys. (1978), 48, 5301.
2. Bierwagen, G.P. Critical Pigment Volume Concentration (CPVC) as a Transition Point in the Properties of Coatings, J. Coat. Tech. (1992), 64(806), 71.
3. Floyd, F.L.; Holsworth, R.M. CPVC as Point of Phase Inversion in Latex Paints, J. Coat. Tech. (1992), 64(806), 65.
4. Eckersley, J.S.T.; Helmer, B.J. Mechanistic considerations of particle size effects on film properties of hard/soft latex blends, JCT Journal of Coatings Technology.
5. Dickie, R.A. Heterogeneous Polymer-Polymer Composites. I. Theory of Viscoelastic Properties and Equivalent Mechanical Models, J. Appl. Poly. Sci. (1973), 17, 45.
For more information, email hugo.denotta@multiquimica.com.
This paper was presented at the 2013 ABRAFATI International Coatings Congress in Sao Paulo, Brazil.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



















