Green Chemistry
Principles and Applications in the Paint Industry













Green chemistry (environmental chemistry or chemistry for sustainable development) is an emerging field with the objective of developing products or industrial processes in an ecologically friendly way. The adoption of this new field of activity in recent years is due to a successful effort in reconciling the interests of chemical innovation with the goals of environmental sustainability and with those of industrial and economic viability.
This article presents general considerations about green chemistry, including historical concepts and 12 principles related to the subject. It also analyzes two paint industry products under the principles of green chemistry: the production of propylene from glycerin produced from biodiesel plants; and the production of butanol from ABE fermentation using sugars from lignocellulosic sources. We apply some of the 12 principles of green chemistry to these processes, since they are relevant for the paint industry. Finally, we draw some conclusions and suggestions for further studies.
Green Chemistry
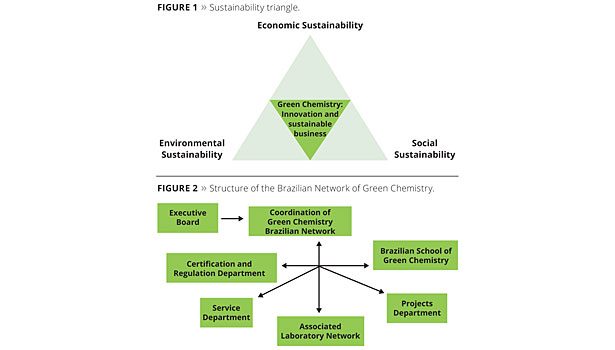
Green chemistry can be defined as chemical routes derived from renewable sources. A holistic approach is adopted, dealing with the use of these byproducts, generating, whenever possible, additional revenue.1 Green chemistry is directly related to the sustainability triangle (Figure 1), with the environmental, economic and social aspects as the pillars of its structure.
History of Green Chemistry
The green chemistry movement started in the early 1990s, mainly in the United States, the UK and Italy, with the introduction of new values and concepts for the different fundamental activities of chemistry and for the corresponding sectors of industrial and economic activities. This proposal was soon extended to involve the International Union of Pure and Applied Chemistry (IUPAC) and the Organisation for Economic Co-operation and Development (OCDE) to establish directives for the development of green chemistry on a global level.2
Several countries have already created initiatives aimed at green chemistry, such as:
- Inter-University Consortium of Chemistry for the Environment, created in 1993 (Italy);
- Green Chemistry Institute, created in 1997 and affiliated with the American Chemical Society in 2001 (USA);
- Green Chemistry Program, of the Environmental Protection Agency (USA);
- Green Chemistry Network, structured by the Royal Society of Chemistry in 1998 (UK);
- Other initiatives in Germany, Australia, Canada, Japan, Spain, Sweden and Russia.3
In Brazil, the Brazilian Network of Green Chemistry (RBQV) is being implemented, as shown in Figure 2.
The Brazilian Network of Green Chemistry’s mission is to play a role and take the responsibility to mobilize and develop mid- and long-term Brazilian scientific and technological competence for generating technological innovations in green chemistry, aiming at the reduction of environmental impacts, and achieving environmental, social and economic sustainability.1
The strategic themes for the RBQV are biorefineries (thermochemical and biochemical routes), alcohol chemistry, sugar chemistry, oil chemistry, phyto chemistry, CO2 conversion and renewable energies.1
The 12 Principles of Green Chemistry
The 12 principles of green chemistry were proposed by Paul T. Anastas, member of the U.S. Environmental Protection Agency (EPA), after the Rio 92 Conference, and are listed below.4
1. Prevention
It is better to prevent waste than to treat or clean up waste after it has been created.
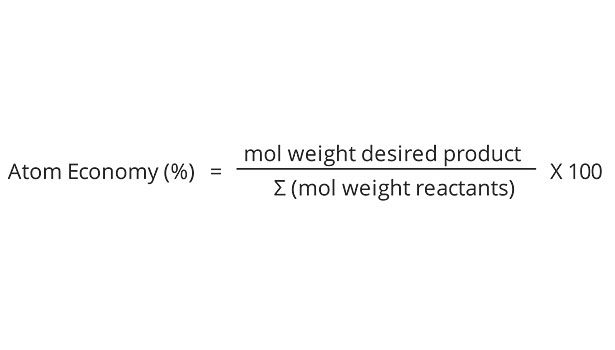
2. Atom Economy
Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
3. Less Hazardous Chemical Syntheses
Wherever practicle, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
4. Designing Safer Chemicals
Chemical products should be designed to achieve their desired function while minimizing their toxicity.
5. Safer Solvents and Auxiliaries
The use of auxiliary substances (e. g., solvents, separation agents and others) should be made unnecessary wherever possible and innocuous when used.
6. Design for Energy Efficiency
Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. If possible, synthetic methods should be conducted at ambient temperature and pressure.
7. Use of Renewable Feedstocks
A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
8. Reduce Derivatives
Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible because such steps require additional reagents and can generate waste.
9. Catalysis
Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
10. Design for Degradation
Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment.
11. Real-Time Analysis for Pollution Prevention
Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
12. Inherently Safer Chemistry for
Accident Prevention
Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions and fires.
Green Chemistry Applications
We chose two relevant products in the paint industry, propylene glycol and butanol, which have great use in numerous sectors of the chemical industry and, particularly, in the paint segment.
Propylene Glycol
Propylene glycol (propane-1-2-diol or 1,2 propanediol) is an odorless and colorless oily liquid, which is hygroscopic and miscible in water, ketone and chloroform. This raw material is used for the production of pharmaceutical products, food, cosmetics, pesticides, solvents, detergents, coatings, etc.5
In the Brazilian market, propylene glycol is used in the manufacture of a large variety of products, including polyester resins, engine coolants, latex paints, heat transfer fluid, de-icing agents, net cleaning agents, lubricants, plasticizers and additives in cement grinding. It is also used in pharmaceuticals, personal care products, cosmetics, food and animal feed.6
In the paint sector, propylene glycol is an important co-solvent of water-based inks for use in architecture, and is also used as an intermediate in the production of alkyd resins for paints and varnishes.6 Propylene glycol contributes to paint protection, helping in the preservation of surfaces, providing stability as an antifreeze agent, protecting buildings against climatic degradation and preserving the quality and beauty of floors in high traffic areas.7
This study examines the production of propylene glycol by petrochemical and glycerochemical routes.
The Petrochemical Route
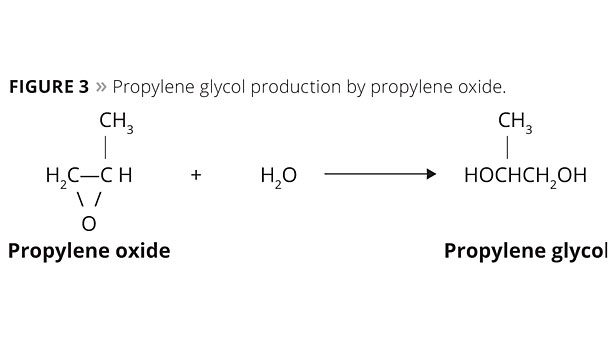
The industrial production of propylene glycol by the petrochemical route uses the process of hydration of propylene oxide. Different manufacturers use non-catalytic processes with temperatures around 473.15 K and pressure of 15 atm5 or catalytic processes at colder temperatures (423.15 K - 453.15 K) in the presence of ion exchange resins or small amounts of sulfuric acid or alkalis. The final product has 20% ??of 1,2-propanediol, 1.5% of dipropylene glycol, and small amounts of other polypropylene glycols. Figure 3 shows the formation reaction of propylene glycol from propylene oxide.8
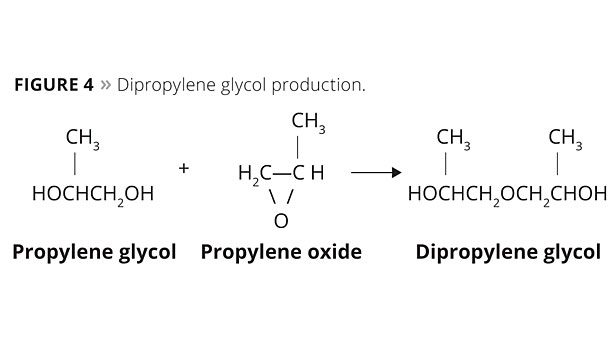
Dipropylene glycol is also obtained in the reaction, but in a lesser amount (Figure 4).
The Glycerochemical Route
In the production of propylene glycol from glycerin derived from biodiesel, hydrogenolysis of glycerin occurs in the presence of a metallic (based on Cu, Ni or Pd) catalyst and hydrogen; its main products are propylene glycol and 1,3-propanediol.9
Figure 5 shows the overall reaction for production of propylene glycol, the co-product 1,3 propanediol, and by-products ethylene glycol, methanol and water.
In Brazil, there is no production of propylene glycol from glycerin on an industrial scale. Studies are still on a smaller scale, and Brazilian companies have a potential market to explore this technology, since there is a large production of glycerin derived from the biodiesel production process. According to information from the National Agency of Petroleum, Natural Gas and Biofuel (ANP), the production of blonde glycerin, with a content of 80% glycerol, in 2012 was approximately 300,000 tons. This value was obtained based on 2012 biodiesel production data from the National Agency of Petroleum, Natural Gas and Biofuel (reaction stoichiometry: 10 wt% to produce crude glycerin and the blonde glycerin, with 80% of glycerin).
Green Chemistry Principles Applied to the Production of Propylene Glycol by the Glycerochemistry Route
Principle 2: Atom Economy
Principle 2, based on the reaction for the formation of propylene glycol (Figure 5), is calculated below:
This efficiency can be even greater considering that the 1,3 propanediol, co-product of the reaction, has commercial value and may be used as a monomer in the manufacture of polyesters or as a chain extender in the production of polyurethanes, lubricants and pharmaceuticals.10
Principle 4: Designing Safer Chemicals
To apply this principle, a survey was conducted with the companies associated with the SITIVESP (Union of Industrial Paints and Coatings of the State of São Paulo) on the use and disposal of propylene glycol.
Of the 55 member companies associated with the SITIVESP, 25 companies responded to the request. Of these, 48% (12 companies) use propylene glycol during the process of producing coatings and resins, but only two companies remain with propylene glycol at the end of the process. In this case the product is stored in drums and then sent for recovery.
Thus, it was proven that, according to the fourth principle, propylene glycol can be considered a safe product as it does not harm the environment and has its waste reused.
Principle 6: Design for Energy Efficiency
Principle 6 can also be easily demonstrated. The route that uses glycerin consists of three basic steps: blonde glycerin purification with temperatures below 473.15 K,11 dehydration of glycerin to ketone at about 573.15 K,12 and finally hydrogenation of the ketone to alcohol, between 473.15 K and 573.15 K.13
On the other hand, the route that uses propylene oxide has as its first stage the pyrolysis of naphtha (a process that occurs between the temperatures of 973.15 K - 1,173 K)14 to obtain propylene. Then the peroxidation or chloridination is made (varies from 298.15 K to 473.15 K),15 producing propylene oxide, which is subsequently hydrated to produce propylene glycol at approximately 473.15 K.5 This temperature refers to a non-catalytic process. If the process is catalyzed, the temperature varies between 423.15 K - 453.15 K.5
The temperature conditions for the stages of production via propylene oxide are higher, mainly because of the pyrolysis of naphtha, implying greater consumption of energy compared to propylene glycol by the glycerochemical route.
Principle 7: Use of Renewable Feedstocks
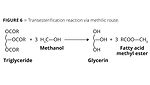
It can be observed that principle 7 is also applicable, since there is the use of glycerin derived from biodiesel plants as a raw material for the production of propylene. As an example, we show the following reaction (Figure 6), where glycerine is a byproduct of the production of biodiesel.
The transesterification consists of the chemical reaction between plant or animal oil (triglyceride) and alcohol (methanol or ethanol) aided by catalysts. The reaction products are methyl or ethyl fatty acid esters and glycerin. At the end of the process, the catalyst is removed through the reaction with hydrochloric acid. Figure 6 shows the transesterification reaction through methilic routes.12
Butanol
Butanol has four isomers (n-butanol, isobutanol, sec-butanol and tert-butanol), and the first two are solvents commonly used in resins and lacquers. The n-butanol, also called n-buty alcohol, is miscible with most common solvents such as alcohols, ketones, aldehydes, ethers, glycols, aromatic and aliphatic hydrocarbons, and has relative solubility in water.
Isobutanol, also known as isobutyl alcohol, is an alcohol with a boiling point of 381.15 K. Low-molecular-weight alcohols, such as butanol, are generally solvents for the manufacture of protective coatings and colorants.
Acetates are the most important esters used in paint formulations and their derivatives, since they are excellent solvents for many natural and synthetic resins such as acrylates, polyurethanes and nitrocelluloses, and are commonly used in solvents for lacquers, lacquer for wood and a large variety of coatings.
Butyl acetate is widely used as a volatile solvent for the manufacture and application of many types of finishes, and is used as a retardant solvent for coatings.16
Butyl acrylate ester, also derived from butanol, is typically polymerized to form acrylic copolymers and terpolymers. It is used in the production of resins, water-based inks, paper coating, impregnation and finishing materials, and adhesives.17
Butanol may be produced by fermentation or petrochemical routes, which will be explained below.
Production of Butanol by Petrochemical Route
Isobutanol and n-butanol are produced by the Oxo process (CO + H2), which involves hydroformylation of propylene, producing n-butyraldehyde or isobutyraldehyde followed by reduction with hydrogen to produce the alcohols, as shown in Figure 7.
Two Brazilian companies, Elekeiroz and Oxiteno, are producing butanol by the petrochemical route. In 2011, the Elekeiroz plant produced 150,000 t/y and the Oxiteno plant produced 10,000 t/y, according to the Yearbook of the Brazilian Association of Chemical Industry.18
Production of Butanol from ABE Fermentation of Biomass
Butanol can be obtained by means of biotechnological processes using biomass from residual sources as a substrate. The fermentation process for obtaining bio-butanol has an output of ketone, butanol and ethanol in the ratio of 3:6:1, respectively.19 In this article, we only examine butanol, the main product of the ABE process.
Figure 8 outlines the route to the production of butanol from the ABE process mentioned above, using the microorganisms Clostridium acetobutylicumand Clostridium tyrobutylicum.
China is leading the world in the production of biobutanol via the fermentation route. In Brazil, the fermentation process uses molasses from sugar cane, but there are other sources of sugar traditionally used in ethanol production, such as sugar beet, cassava, corn and lignocellulosic residues like sugarcane bagasse, rice straw, wood chips, and so on.
Principles of Green Chemistry Applied in the Production of Butanol by Fermentative Route
Principle 4: Designing Safer Chemicals
To apply principle 4, a survey was conducted with companies associated with SITIVESP about what is done with the butanol residue at the end of the production process of inks.
Among the 25 respondent companies, the majority mentioned that there is no generation of waste in the process. Only one company stores butanol in tanking systems and then the product is shipped for recovery. Another company said that the remains of butanol are distilled and reused in the following resins. Therefore, in general, the solutions found by the market are in accordance with the fourth principle: the production of butanol does not cause damage to the environment because its residues are recycled.
Principle 6: Design for Energy Efficiency
Principle 6 can also be easily demonstrated. The range of agro-industrial residues that can be used as a substrate to the fermentation process is immense, from sugars derived from sugar cane (sucrose) and sugars (glucose and xylose) present in lignocellulosic residues. Obtaining the sugars derived from lignocellulosic wastes involves chemical and biochemical processes of deconstruction of lignocellulosic fibers to release these sugars.
The next step is fermentation, which is the use of bacteria for the substrate (sugars) to obtain bio-butanols in low-temperature conditions, around 303.15 K to 308.15 K.19 On the other hand, the petrochemical route uses hydroformylation of propene (temperature between 373.15 and 473.15 K) and then performs the hydrogenation step that occurs close to 388.15 K, in the presence of nickel catalyst.20
The process conditions using the petrochemical and biochemical routes are completely different. It is observed that fermentation conditions to obtain butanol are less stringent than the conditions for the petrochemical route.
Principle 7: Use of Renewable Feedstocks
It can be observed that principle 7 is also applicable, since sugars are used from the agricultural industry as raw materials from renewable sources for the production of butanol.
Conclusions and Suggestions
This study examined alternative routes for the production of butanol and propylene glycol. It was found that it is possible to produce these important solvents by routes closer to the principles of green chemistry and therefore less harmful to the environment than the traditional processes.
The production process of propylene glycol from glycerin derived from biodiesel production stood out because of the high energetic and atomic efficiency, use of safe products, and reduced waste generation, besides the use of renewable raw materials.
On the other hand, butanol produced by the ABE fermentation process showed an alternative of greater energy efficiency, use of safe products and raw materials from renewable sources.
In a world of changes, green chemistry can be seen as an innovation driver and a sustainable business in various industries like the paint market. In Brazil, the seventh principle of green chemistry (use of renewable feedstocks) stands out as a great strategic opportunity for the country.
Several factors, such as the potential of industrial biology, environmental restrictions on the use of fossil raw materials, guidelines of business strategies and perspective of technological innovation can be viewed as determinants of the market for the use of renewable raw materials for the process industries. These factors suggest that innovations based on the development of bio-processes and bio-products should have an important weight in the industry during the 21st century.
As a continuation of this study, a life cycle analysis of the solvents studied is recommended. In addition, an analysis of management tools of green chemistry and green chemistry metrics applied to the paint segment could also be carried out. n
References
1 Sousa-Aguiar, Eduardo Falabella. Química verde: um novo conceito energético baseado na economia ecológica do futuro. Rio de Janeiro. Cenpes, 2013.
2 Assunção, F.C.R. Química Verde no Brasil 2010-2030, Ed. rev. e atual. Centro de Gestão e Estudos Estratégicos. Brasília, DF, 2010.
3 Ludovico, F. Introdução a Química Verde – Conceitos e aplicações. Rio de Janeiro. PUC. 2013.
4 Mulvihill, M.J., et al. Green Chemistry and Green Engineering: A Framework for Sustainable Technology Development; August, 2011; EUA.
5 Chinn, H; Kumamoto, T. Propylene glycols. 2011, Chemical Economics Handbook-SRI Consulting.
6 Dow, Technical Data Sheet: Propylene Glycol, Dow Industrial Grade. Available at: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_0042/0901b80380042aa5.pdf?filepath=propyleneglycol/pdfs/noreg/117-01542 pdf&fromPage=GetDoc. Accessed on June 12, 2013.
7 Propylene Glycol, Available at: http://www.propylene-glycol.com/index.php/industrial-propylene-glycol/paints-coating. Accessed on May 22, 2013.
8 Chemindustry. 1,2-propanediol. Available at: http://chemindustry.ru/1,2-Propanediol.php. Accessed on June 10, 2013.
9 Mota, C.J; Pestana, C.F.M. Co-produtos da Produção de Biodiesel; Revista Virtual de Química, vol. 3 núm. 5, p. 416- 425. October 2011.
10 Barbirato, F; Himmi, D.H; Conte, T; Bories, A. 1,3 propanediol production by fermentation: An interesting way to valorize glycerin from the ester and ethanol industries. 1996, INRA, Gruissan, França.
11 Mendes, D.B; Serra, J.C.V. Glicerina: uma abordagem sobre a produção e o tratamento; Revista Liberato, v.13, n.20, p.01-134, July/December 2012, Novo Hamburgo.
12 Mota, C.J.A.; Silva, C.X.X.; Gonçalves, V.L.C. Gliceroquímica: novos produtos e processos a partir da glicerina de produção de biodiesel. Química Nova, v. 32, n. 3, p. 639-648, March 2009.
13 Petrobras, B.; Everett, J.; Klotz Rabello, C.R. Production of propylene glycol from glycerol. Petroleo Brasileiro S.A. WO2011104634 A2, 24 February 24, 2011, September 1, 2011. Available at: http://www.google.com/patents/WO2011104634A2?cl=en. Accessed on May 31, 2013.
14 Faria, R.M.B. Uma metodologia de avaliação de catalisadores de hidrogenação de gasolina de pirólise. Universidade Federal do Rio de Janeiro, Rio de Janeiro, 2011.
15 Yamamoto, J.; Goto, S. Método para producir óxido de propileno. Sumitomo Chemical Company, Limited. C07D301/19, April 18, 2012. Available at: http://patentados.com/patente/metodo-para-producir-oxido-de-propileno.2/ Accessed on May 31, 2013.
16 Fazenda, J.M.R.. Tintas – Ciência e Tecnologia; 4ª edição Rev. e Ampl.; São Paulo; Editora Blücher; 2009.
17 Brancotex, Indústrias Químicas LTDA. Available at: http://publy.floot.com.br/brancotex/produtos_detalhe.php?cod=108&cat=21&tipo=14. Accessed on June 3, 2013.
18 Associação Brasileira da Indústria Química – ABIQUIM. Anuário da Indústria Química Brasileira; Edição 2012, p.122 – 123. São Paulo, 2012.
19 Yu Sing Jang, et al. Butanol production from renewable biomass by clostridia. Bioresource Technology. n.123 ; pgs. 653-663. 2012.
20 Pucrs. Hidroformilação de Alquenos – Processo “Oxo”. Available at: http://www.pucrs.br/quimica/professores/arigony/hidro.html. Accessed on June 4, 2013.
21 BASF and Oleon celebrate grand opening of propylene glycol production plant; Ludwigshafen, June, 28, 2012. Available at: http://www.basf.com/group/pressrelease/P-12-310. Accessed on May 22, 2013.
22 Colodette, J.L. Produção de Etanol de Materiais Lignocelulósicos. Departamento de Engenharia Florestal, Universidade Federal de Viçosa. Viçosa, MG, 2012.
23 Fernando A.S; et al; Potencial da palha da cana-de-açucar para produção de etanol; Química Novav.35 n.5, São Paulo, 2012.
24 Garbelotto P. Solventes Industriais – seleção, formulação e aplicação; Editora Blucher; 1ª edição; 2007.
25 Green chemistry; Available at: http://www.adm.com/en-US/products/evolution/Propylene-Glycol/Pages/Green_Chemistry.aspx . Accessed on May 22, 2013.
26 Hawkins, et al; Methods for the economical production of biofuel from biomass. Gevo, Inc. US8431374 B2, April 30, 2013. Available at: http://www.google.com/patents/US8431374. Accessed on June 6, 2013.
27 Oppe, E.E.G; Salvagnini, W.M; Taqueda, M.E.S. Redução da demanda energética na desidratação da glicerina obtida a partir do biodiesel; Escola Politécnica de Engenharia Química, Universidade de São Paulo, October, 2007.
28 Pinheiro, F.G.C, et al; Pré-tratamento termoquímico do bagaço da cana-de-açucar para a produção de açucares fermentescíveis. II Simpósio Internacional sobre Gerenciamento de Resíduos Agropecuários e Agroindustriais. Foz do Iguaçu. PR, 2011.
Acknowledgments
The authors thank Claudio Gabriel Pinheiro Geraldino, Gabrielle Viana Dutra and Ywrrenan Cardoso Amorim, from Petrobras Distribuidora S/A, Airton Sicolin, from SITIVESP (Union of Industrial Paints and Coatings of the State of São Paulo), and Evan Beach, from Center for Green Chemistry and Green Engineering of Yale University (USA), for the partnership and contribution to this study.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!
















