Effective Functional Additives
for Low- to Zero-VOC Coating Formulations









Over the past several decades, the market share for waterborne coatings has increased at the expense of solventborne formulations. There were several drivers for this shift, but one of the primary motivations was a conversion to a more environmentally friendly medium in formulations. These waterborne formulations were still typically supplemented with other co-solvents to address some of the issues associated with utilizing water-based dispersions. For example, glycols were included to depress the freezing point of the water to protect the formulation in the event of exposure to freezing conditions, and to slow the evaporation of water during film formation in an effort to provide more robustness in the final application. Volatile coalescents were also used to temporarily depress the glass transition temperature of the higher-molecular-weight polymers in the latex to allow for adequate film formation at lower temperatures while still ultimately yielding a hard, resistant final film.
However, there is now an on-going push from government agencies and consumers to further mitigate health and environmental impact concerns by reducing the associated VOC content of waterborne formulations. This implies a significant reduction or elimination of glycols and volatile coalescents from formulations. Although the desire would be to provide low-VOC coatings with equivalent to superior performance as compared to higher-VOC predecessors, satisfactory alternatives to the co-solvents that delivered the key properties alluded to above have proven elusive to date. To better comprehend some of these inherent challenges, it is instructive to consider the underlying fundamental mechanisms.
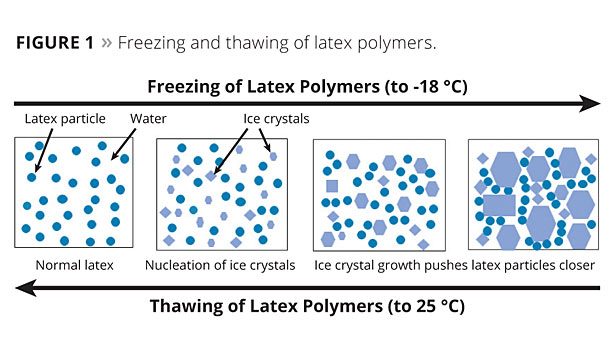
In a latex-based dispersion or formulation, the freezing process starts with the formation of some initial ice crystals. As these ice crystals grow or additional ice crystals form, the net reduction in the density of the water effectively increases the volume solids of the dispersion and forces the latex particles to pack closer together. Since the initial ice that forms will be low in salt content, the electrolyte concentration in the remaining liquid water will increase, which compresses the protective double layer of electrostatically stabilized lattices. These combined factors can reduce initial latex stability and lead to particle-to-particle contact. If latex stability is not sufficient under these conditions, this aggregation is not reversible upon thawing and results in undesirable coagulation. Since polymer flow and particle coalescence can occur at these low-temperature conditions, this situation is further exacerbated with the lower Tg or softer binders utilized in many low-VOC paints. Therefore, without the use of glycols to reduce the formation of ice crystals in freezing conditions, many low-VOC waterborne paints fail to provide sufficient freeze/thaw (F/T) stability (Figure 1).
Another important property of waterborne coatings that is significantly impacted by the reduction or removal of glycol from the formulation is open time. The open time of a coating is the period of time during which the wet paint remains workable after it has been applied to a substrate, allowing for corrections, through re-brushing or rolling, without resulting in surface defects. In an emulsion-based paint, this time concludes in the film formation process once the latex particles have made contact and begun to coalesce, since at this point the water from any additional paint applied is not able to re-disperse the particles to yield new flow. This time is considerably shorter than the film drying time, which is based on the completion of particle coalescence. Since the lower-molecular-weight polymers in solvent-based formulations can be more easily re-dispersed after initial application, even traditional waterborne formulations have been relatively deficient in open time performance. Without the use of glycols to slow the evaporation of water from the film to sufficiently delay initial particle-to-particle contact, low-VOC paint formulations are fundamentally even more challenged in this respect.
In this study, two new additives designed to help address these challenges are presented. The FT additive targets improvement in freeze/thaw stability of low- to zero-VOC binders and latex paints. The effects of the FT additive on the F/T stability of a waterborne paint were investigated and will be discussed. The OTE additive targets an increase in open time of low- to zero-VOC binders and latex paints. The effects of the OTE additive on the open time extension of a waterborne paint were investigated and will be discussed.
The challenge was to identify an approach for latex paints that met low- to zero-VOC requirements that yielded F/T stability and improved open time without compromising overall film performance. In addition to F/T stability and open time, benefits to other paint qualities such as gloss and wetting are also discussed.
Experimental
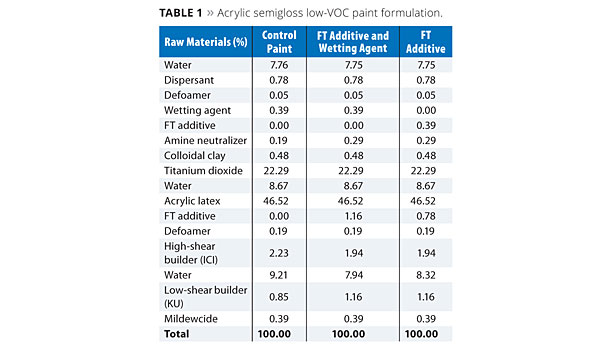
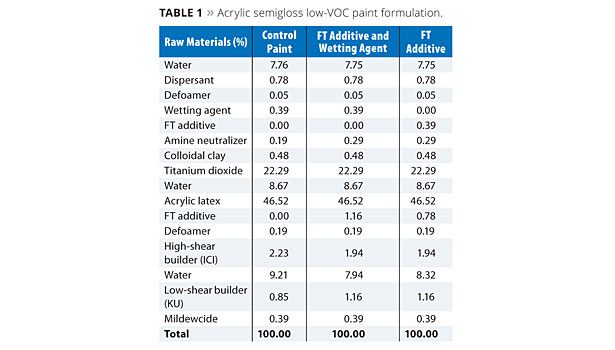
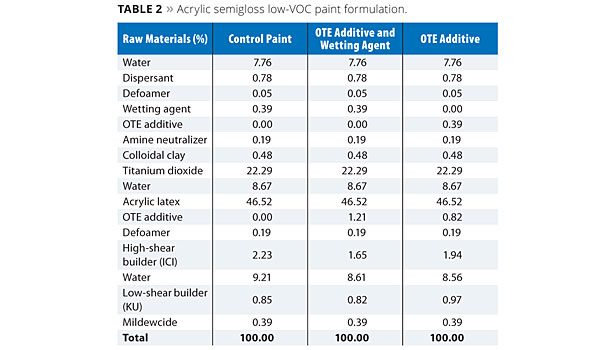
The additives FT and OTE were evaluated at a usage level of approximately 1% (based on total formula weight) in a low-VOC acrylic semigloss paint formula as shown in Tables 1 and 2, respectively. In each case, a control formula was compared to prototypes containing the FT or OTE additives both with and without a conventional wetting agent. For the prototypes without the wetting agent, a portion of the respective FT and OTE additives was shifted to the grind and substituted as a replacement on a weight basis without increasing their overall usage levels in the formulation.
Paint Formulations
Paint formulations were prepared as outlined in Tables 1 and 2. All evaluations on the coating formulations were performed at least 24 h after the initial preparation of the paints.
Results and Discussion
FT Additive
Results for the studies involving FT additive are shown in Table 3. The Control Paint failed after the initial F/T cycle, while addition of FT additive to the prototypes, either with or without wetting agent, produced paints that demonstrated no evidence of coagulation through five cycles of F/T testing. Furthermore, a minimal increase in viscosity was observed with these two prototypes after the five cycles of F/T testing. The level of FT additive in this formulation is a suggested starting point but cannot be generalized for all formulations. The amount of additive required for desired performance in a specific formulation will be dependent upon a number of factors such as binder chemistry, binder particle size, paint PVC, levels of other additives and reagents used in the formulation, etc.
In addition to freeze/thaw stability, other key performance paint properties were also evaluated and are listed in Table 3. The results indicate that FT additive generally had no significant impact on block, scrub or stain resistance, surfactant leaching, or low-temperature cure. However, the additional dispersion stability resulted in an increase in film gloss for each of the prototypes. FT additive also allowed for the removal of the conventional wetting agent used in the formula without significantly impacting key paint performance properties, demonstrating potential multifunctional benefits.
Results and Discussion
OTE Additive
Results for the studies involving OTE additive are shown in Table 4. The Control Paint exhibited poor open time while addition of OTE additive to the prototypes, either with or without wetting agent, produced paints that yielded significantly better open time, as shown in Figure 2. The level of OTE additive in this formulation is a suggested starting point, but cannot be generalized for all formulations. The amount of additive required for desired performance in a specific formulation will be dependent upon a number of factors such as binder chemistry, binder particle size, paint PVC, levels of other additives and reagents used in the formulation, etc.
In addition to extended open time, the incorporation of the OTE additive affords improvement in other coating properties, summarized in Table 4. Analogous to the use of the FT additive, improved colloidal stability results in an increase in film gloss with each of the prototypes. In the same vein, freeze/thaw stability is also improved, though is not quite as robust under the same conditions relative to the FT additive based on viscosity rise. Scrub resistance results indicate that negative impacts to water sensitivity can be minimized in this paint through optimization of the formulation, e.g., removal of the conventional wetting agent enabled by functional replacement with the OTE additive.
Additional Applications for OTE Additive
OTE additive was also found to be an effective open time extender for industrial coatings and other similar high-film-build applications. In addition to architectural paints, OTE additive was found to be an excellent retarder (prevention of ink drying on the screen) for water-based screen printing inks, thus enabling the replacement of plasticizers. Additional applications and functional benefits continue to be studied.
Conclusions
Two new additives, FT and OTE, have been developed with patented applications for improving performance in solvent free, coatings. Both of these products are APEO free and solvent free, and can be easily incorporated into waterborne formulations. With the low-VOC acrylic semigloss formulation in this study, the additives provided either a significant enhancement in freeze/thaw stability or a marked increase in open time. Additional multifunctional benefits such as improved gloss and pigment wetting were also observed, enabling the development of higher-performance low-VOC coatings.
References
1. Blackley, D.C. Polymer Latices-Science and Technology, 2nd Ed., 1, Chapman & Hall, 1997.
2. Bosen, S.F.; Bowles, W.A.; Ford, E.A.; Person, B.D. Antifreezes, Ullmann’s Encyclopaedia of Industrial Chemistry, 5th Ed., A3, VCH Verlag, 1985.
3. U. S. Patent: US 5,399,617.
4. U. S. Patent: US 6,933, 415 B2.
5. Holl, Y. et al. Film Formation in Coatings, Mechanisms, Properties and Morphology; Urban, T.P.M.W., Ed., 2001.
Sidebar:
Evaluation of Standard Paint Properties
ASTM D562-10 – Standard Test Method for Consistency of Paints Measuring Krebs Unit (KU) Viscosity Using a Stormer-Type Viscometer
ASTM D4287-00(2010) – Standard Test Method for High-Shear Viscosity Using a Cone/Plate Viscometer
ASTM D1475-98(2012) – Standard Test Method for Density of Liquid Coatings, Inks and Related Products
ASTM D4062-11 – Standard Test Method for Leveling of Paints by Draw-Down Method
ASTM D4400-99(2012)E1 – Standard Test Method for Sag Resistance of Paints Using a Multinotch Applicator
ASTM D2805-11 – Standard Test Method for Hiding Power of Paints by Reflectometry
Color Acceptance: Rub-Up
Side-by-side drawdowns (test/control) on Leneta Form 1B; (sealed/unsealed charts)
.006 Bird Applicator – Ambient dry overnight
Color Values: BYK Gardner Color-Guide 45°/0° geometry, D65/10°, CIELab color system
Gloss (60°)/(85°)
Side-by-side drawdowns (test/control) on Leneta Form 1B; (sealed/unsealed charts)
# 7 DOW Applicator – Ambient dry overnight - BYK Gardner Micro-TRI-Gloss Gloss Meter
ASTM D2486-06(2012)E1 – Standard Test Methods for Scrub Resistance of Wall Paints, Gardco scrub tester (Paul N. Gardner Co.)
ASTM D4828-94(2012)E1 – Standard Test Methods for Practical Washability of Organic Coatings, Gardco scrub tester (Paul N. Gardner Co.)
ASTM D2243-95(2008) – Standard Test Method for Freeze/Thaw Resistance of Waterborne Coatings
Freeze/Thaw Stability Testing
Freeze/thaw stability of the latex paints was measured by ASTM D2243-95. Before measuring the viscosity, the thawed paints were well mixed by hand using a spatula.
Open Time Evaluation
Open time was evaluated according to ASTM D7488-10. A black vinyl Leneta scrub panel that was secured on an aluminium drawdown plate in a constant humidity/temperature room (CTCH) was used as the substrate. The Leneta scrub panel was divided into 8 horizontal sections. A 250 µm wet film thickness paint was drawn down using a Dow film caster. On each section, a mark (X) was made immediately after casting the paint, using the handle tip of the brush. Test paint was then applied in perpendicular sections, brushing each section across the initial casted section, in one direction. The perpendicular sections were repeated at 2 min time intervals using the same number of brush strokes for each time interval. The coating was allowed to dry in the CTCH room for 24 h prior to rating the results.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!