New Titanium Dioxide Process







Titanium dioxide (TiO2) production has existed for almost 100 years. People use this principal white pigment every day, as it is employed in paint, plastics, food and beverages, cosmetics, textiles and paper. Global demand for TiO2 in 2012 was 5.3 million tonnes (8 lb per capita in North America).
Article Index:
- The AT Process
- Pure Rutile TiO2 via the AT Process
- Properties Achieved from the AT Process
- Surface Treatment
- Conclusion
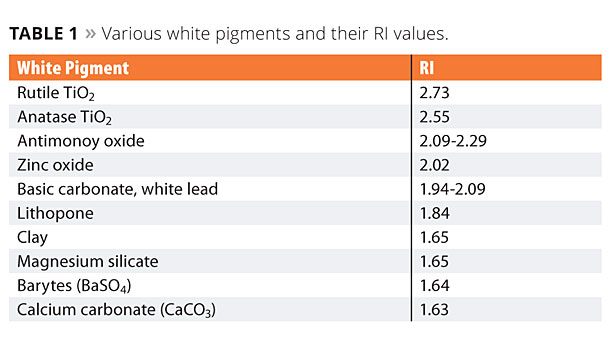
As a pigment, TiO2 is dispersed in various matrices in order to allow scattering and reflection of visible light. For many years, different substances, characterized by their Refractive Index (RI), have been used to achieve light reflection – the higher the RI, the higher the reflection. TiO2 has the highest RI among white pigments typically used in industry (Table 1). The scattering of visible light gives TiO2 its most important optical properties, including opacity, brightness, gloss, tone (white) and undertone (color), lightening power (tinting strength), weather resistance, durability and abrasiveness.
Until now, TiO2 has been produced by either the sulfate process (1916) or the chloride process (1948). Each process has its own strengths and weaknesses. Argex Titanium Inc., a Canadian company, has recently transitioned from a mining exploration company with assets in Labrador, to a near-term TiO2 producer with the development of a third TiO2 process called Argex Technology (AT). This proprietary and patented hydrometallurgical process, based on the CTL solvent extraction technology, uses solvent extraction (SX). SX, a metallurgical process (liquid-liquid extraction), is a well-known technology used to separate compounds. It has been used for many years for several applications, such as uranium production.
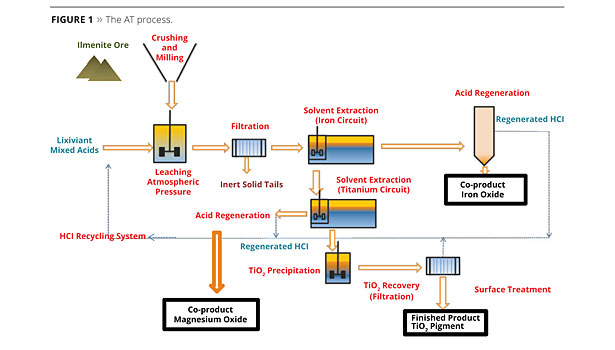
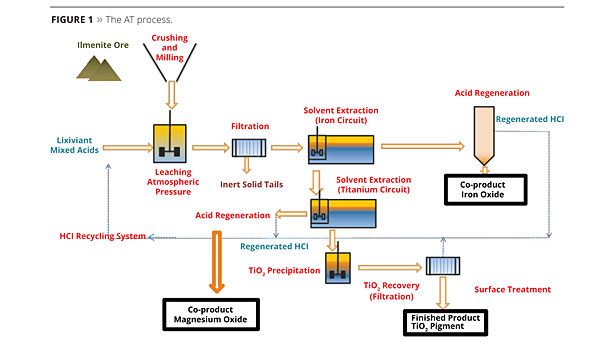
The AT Process
During the AT process, ilmenite ore is leached by acids, followed by a first solvent extraction to remove iron, and then a second solvent extraction to remove TiOCl2 and to achieve a clear solution containing titanium. A hydrolysis onto this clear solution, without impurities and contaminants, will result in pure TiO2. The pigment washing steps in the AT process are easier and more efficient compared to the sulfate process (Figure 1).
Inexpensive and available TiO2 feedstock is used as raw material, supplied by conventional ore suppliers (i.e., ilmenite); also used are “tailings” (lower cost) from the mining industries, which are raw materials that other processes cannot use. As a cost-effective process, AT is environmentally attractive, as it is energy efficient, does not use high pressure or temperatures, or chlorine, is a closed-loop operation and has very low inert tailings that are easy to recover. All by-products have a high level of purity and are easily re-used in various industries like water treatment. The AT process represents a new step in environmental sustainability and an improvement in the carbon footprint for the TiO2 process.
This efficient process is being conducted at the current Argex pilot plant, continuously running at Salaberry-de-Valleyfield, Quebec. The first production unit of 50,000 mt will also be at this location. The existing industrial infrastructure on a brownfield site saves time and large capital expenditure, and can accommodate additional TiO2 production modules, offering significant potential for expansion. The proximity to major port, rail and highway infrastructures facilitates the shipping of raw materials and merchandise via ground and maritime routes. The region is already favored by the hydrometallurgical and chemical processing industries with proximity to HCl acid production, access to natural gas supplies, close proximity to Hydro Quebec’s Beauharnois generating station, and proximity to Montreal’s Trudeau International Airport. The Valleyfield plant will be commissioned by early 2015, and will be in production during the first half of that same year.
Pure Rutile TiO2 via the AT Process
The chloride process starts with chlorination of the ore at a temperature of around 1000 °C in a fluidized bed reactor in the presence of coke. Coke is a fuel with few impurities and a high carbon content, usually made from coal. It is the solid carbonaceous material derived from destructive distillation of low-ash, low-sulfur bituminous coal.
The sulfate process precipitates TiO2 by hydrolysis from a solution (the black liquor) made by leaching ilmenite (FeTiO3) with sulfuric acid (H2SO4) and trace amounts of metals, particularly chromium (Cr), vanadium (V) and iron (Fe), which impact product color and give it a yellow tone (Figure 2).
Each of the two processes has trace amounts of impurities before achieving final solid TiO2 particles. These impurities can influence the morphology, particle size and the color tone of the pigment. With its two solvent extraction steps, the AT process precipitates TiO2 from a clear solution without impurities (Figure 3).
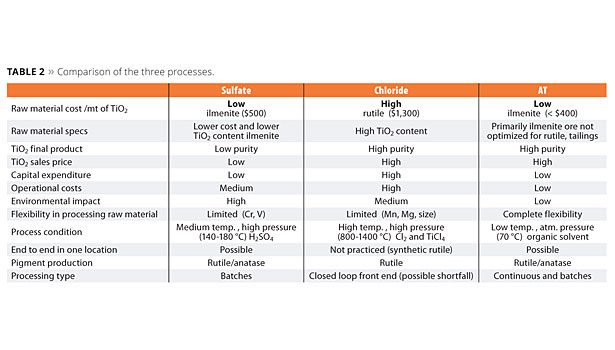
The three TiO2 production processes have great differences. The AT process demonstrates a great improvement in terms of quality and cost, and it is the most environmentally friendly (Table 2).
Properties Achieved from the AT Process
Whiteness can be characterized by parameters such as hiding power (opacity), brightness and color tone. In coatings, these properties will only be developed if the TiO2 pigment is perfectly dispersed in the matrix.
Hiding power mainly depends on light scattering. A typical human eye responds to wavelengths from about 400 to 700 nm. A light-adapted eye generally has its maximum sensitivity at around 555 nm. Wavelength scattering becomes more efficient when the particle has a diameter of about half the size of the incident wavelength. TiO2’s most efficient scattering power is achieved with a particle size weight average of approximately 0.28 µm. Other critical parameters include particle design (the more spherical the better) and particle size distribution (the narrower the particle size distribution, the better). With the AT process, TiO2 particles are created, after the two solvent extractions, from a pure TiOCl2 solution. The precipitation that takes place without impurities of other metal ions in the crystal lattice of the titania, such as Fe, Cr and V, will permit better control of the particle size design and distribution.
Brightness mainly depends on the purity of the pigment. Again, because of the two solvent extraction steps in the AT process, the TiO2 pigment obtained will contain less coloring trace element than the chloride or sulfate processes.
Color tone can be regarded as the identity of the pigment. Two values are to be considered: the tone (in white), which depends more or less on the weight average of the particle size, and the undertone (in grey), which depends on the particle size distribution. When dispersed into a paste of colored pigments, the resulting color can be different, depending on the batch of TiO2, as shorter and longer wavelengths scatter differently. TiO2 that has a coarser particle size distribution scatters red and green wavelengths preferentially, while a finer particle size distribution scatters the blue wavelengths better.
Because a grey color with a bluish tone appears more fresh to the human eye, and a yellowish tone has a stained appearance, TiO2 with a bluish tone is preferred. However, we must remember that a fine particle size distribution, meaning a bluish tone, will lead to a loss of scattering power for longer wavelengths (e.g., red), which means a reduction in the total hiding power or opacity.
In the current chloride and sulfate processes, TiO2 particles are rarely identical from batch to batch, sometimes giving large undertone specifications. With the AT process and its proprietary solvent extraction steps, it is easier to control the particle size distribution.
Surface Treatment
Obtaining pure and very well distributed TiO2 particles is necessary, but not sufficient – particularly in the coatings industry, where pure TiO2 particles need to have some surface treatments, such as organic treatment to improve flowability and dispersion in the binder, and inorganic treatment to improve stability and light resistance (durability).
Pigment dispersion is a priority. In a bad dispersion, even the best pigment will not be able to develop its own optical properties. All pigments can, in theory, be well dispersed – it is only a question of time and/or the use of chemical aids. However, this raises the cost.
As a new TiO2 producer, we do not have the constraints of an existing installation to mandate the surface treatment methods, and our materials will propose the latest available technologies, such as alumina plus dense skin silica and/or zirconium. This technology is being developed with the collaboration of PPG.
Three pigment series will be made available quickly:
- RGX™ 100 series: untreated rutile for cosmetics, food, medical and paper;
- RGX™ 200 series: treated rutile for plastics; and
- RGX™ 300 series: treated rutile for coatings.
Conclusion
Today the titanium dioxide industry faces great change and challenges. A century after the first industrial production with the sulfate process and 60 years after the birth of the chloride process, the AT process brings a new facet to this industry. With better flexibility using raw materials, cost-efficient and environmentally friendly features, Argex will be able to bring to the market a TiO2 pigment that will better fulfill the current and future requirements of industries. NDAs are in place with major end users and distributors, and more collaboration and purchase agreements are to come.
For more information, visit www.argex.ca.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!