Influence of Nanoclay on Water and Corrosion Resistance in Polyurethane Coatings, Part 2









Nanomaterials have a relatively large surface area. As particles decrease in size, a greater number may be found at the surface, creating a more reactive surface than achieved with larger-size particles. Since nanoparticles provide a larger surface area and quantum effects, it is expected that replacing existing larger-size extenders with nanosize extenders could provide better coating properties. Part 1 of this article (PCI, January 2013 issue, page 44) revealed that incorporating nanoclay into polyurethane coatings significantly improved dry adhesion as well as high-pressure hot-water-spray test properties. This article discusses the same systems tested for water, humidity and corrosion resistance.
Experimental
Synthesis of Nanoclay-Based Coatings
Nanoclay, which is organically modified nanodispersable layered silicate with an average particle size of 25 nm (Al2O3, 2SiO2, 2H2O), was used for the study. The synthesis of nanoclay coatings was carried out in two steps.
-
Nanoclay Dispersion
Generally, nanoparticle dispersion is extremely difficult due to the large surface area. Polyurethane coatings were selected because of their excellent durability. Acrylic polyol (80% on solids) and polyester polyol (20% on solids) were used as dispersing resins. The following samples were dispersed until a fineness of gauge less than 20 microns was achieved: 0% nanoclay; 2% nanoclay; and 5% nanoclay. -
Coating Preparation
The above dispersions were mixed with trimer isocyanate separately; the OH-NCO ratio was maintained around 1:1.5 for all experimental samples. Then all the samples were sprayed directly over mild steel and aluminum substrates, and finished with a solventborne topcoat.
Test Methods and Results
The following properties were evaluated after seven days of curing.
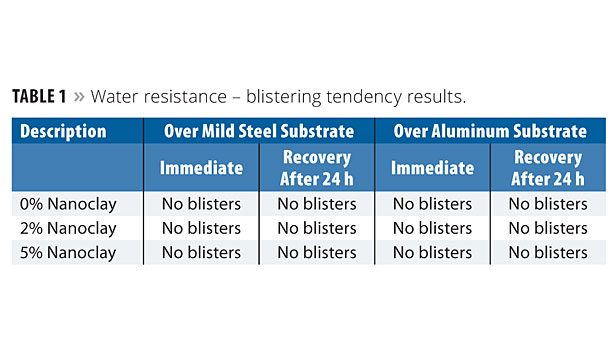
Water Resistance
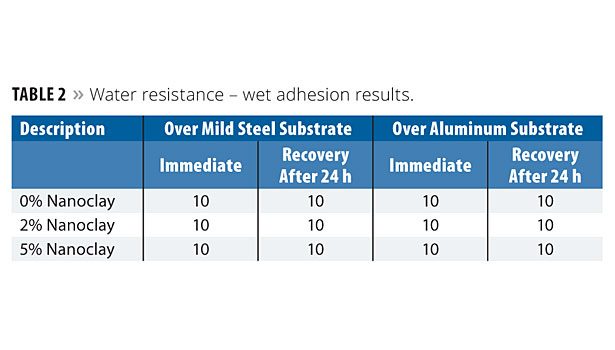
The nanoclay-based coatings applied over mild steel and aluminum substrates were immersed in DM water at a temperature of 25±1 °C for a period of 10 days. The panels were then removed and evaluated for blisters, shrinkage and adhesion. The panels were evaluated again after 24 h. Both the immediate and 24 h results are reported. The blistering results are shown in Table 1. Blistering was determined according to ASTM D 714; blister ranking: D-Dense; MD-Medium Dense, F-Few; W-Wrinkling. Wet adhesion results are shown in Table 2. The incorporation of nanoclay at 2% and 5% concentration levels exhibited a similar level as that of the 0% level of nanoclay. This means that the addition of nanoclay does not affect the coating film properties in terms of blistering or adhesion properties.
Humidity Resistance
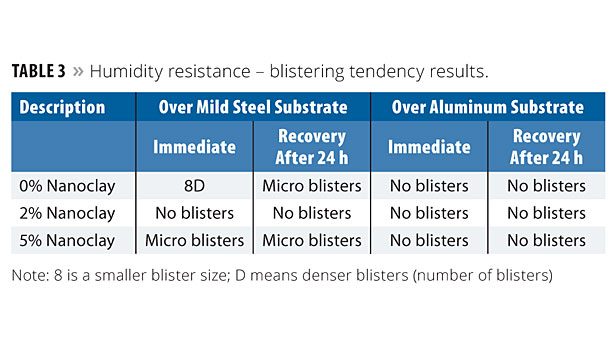
The panels were subjected to a Cleveland condensing humidity tester where the temperature was 38-40 °C and the relative humidity was about 95%. The panels were removed after 10 days of continuous exposure and evaluated for blisters, shrinkage and adhesion. The panels were evaluated again after 24 h. Both the immediate and 24 h results are reported. Blistering was determined according to ASTM D 714; blister ranking: D-Dense; MD-Medium Dense, F-Few; W-Wrinkling. Table 3 shows the blistering results. The results show improvement with the addition of nanoclay, especially over the mild steel substrate. The panels with 2% nanoclay had no blistering tendency, whereas slight microblisters were observed with 5% nanoclay. No significant influence was observed over the aluminum substrate.
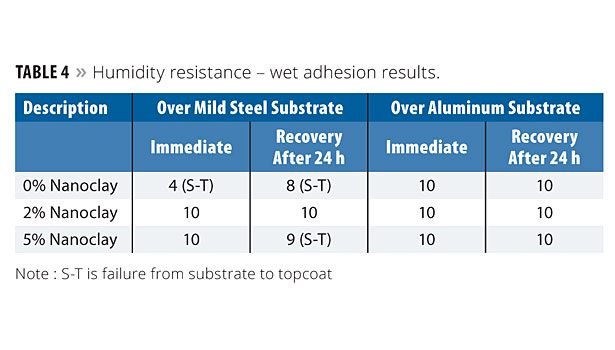
Table 4 shows the wet adhesion results. The wet adhesion properties showed tremendous improvement with nanoclay, especially over the mild steel substrate. As with the blistering, the 2% nanoclay sample was completely intact on the substrate, whereas negligible failure was noticed with the 5% sample. However, both concentration levels of nanoclay exhibited better adhesion properties than the 0% level. There was no significant influence noticed with the aluminum panels.
Salt Spray Corrosion Resistance (ASTM B 117)
All experimental samples applied over mild steel substrate were subjected to testing in a salt spray test chamber. The salt spray was executed at an air pressure at the nozzle of 1 to 1.3 bar. Five parts of sodium chloride were dissolved in 95 parts of demineralized water, and the quality was maintained as per ISO 3696. The temperature in the cabinet was kept at 35 ± 2 °C. After 800 h, all the panels were removed and evaluated for blistering and corrosion. Blistering was determined according to ASTM D 714. The distance of corrosion/delamination along the scribe (one side) is reported in mm’s. The results are shown in Table 5. All experimental samples showed a similar level with respect to blistering and corrosion propagation. Nanoclay addition did not have any adverse effect in the salt spray test.
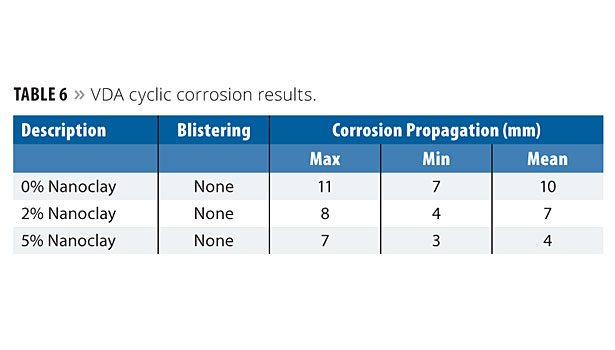
VDA Cyclic Corrosion Resistance
All samples applied over mild steel substrate were subjected to testing in a VDA test chamber. It is useful to test vehicle components using cycles of changing corrosive effects in accordance with VDA 621-415. The equipment is similar to that of salt spray, which is provided with a program to achieve the prescribed cycle of condensation as shown below:
- Salt spray (5% NaCl solution): 24 h
- Condensation at 38 °C: 8 h
- 23±2 °C and relative humidity: 16 h
After five cycles, all the panels were removed and evaluated for blistering and corrosion propagation. The results are shown in Table 6. No blistering was observed, even after five cycles (850 h). The corrosion propagation results showed improvement with nanoclay. However, a significant improvement is noticed at 5% concentration level. This result clearly ensures that closely packed platy nanoclay has a tendency to protect metal from corrosion.
Acetic Acid Spray Corrosion Resistance
(ISO 9227, ASTM G85)
Samples applied over aluminum substrate were subjected to testing in an acetic acid spray test chamber. The salt spray was executed at an air pressure at the nozzle of 1 to 1.3 bars. Five parts of NaCl were dissolved in 95 parts of demineralized water, and the quality was maintained as per ISO 3696. Glacial acetic acid was used to adjust pH from 3.1 to 3.3; cabinet temperature was kept at 35 ± 2 °C. After 800 h, all the panels were removed and evaluated for blistering and corrosion propagation; blistering was determined according to ASTM D 714. The results are shown in Table 7. The results show that the blistering tendency is almost similar in all the samples. The corrosion resistance properties over the aluminum substrate improved significantly with nanoclay. The corrosion level without nanoclay went up to 20 mm, whereas with nanoclay it reached a maximum level of 2 mm.
Conclusion
The study reveals that incorporating nanoclay improves humidity resistance, VDA cyclic corrosion over mild steel substrate and acetic acid spray corrosion over aluminum substrate. Water resistance and neutral salt spray corrosion results showed that the nanoclay had no significant effect. Overall, it can be concluded that incorporating nanoclay is an option for coating formulators to enhance durability performance – especially corrosion resistance properties.n
Acknowledgement
The authors would like to acknowledge Dr. Sudhakar Dantiki, Managing Director AkzoNobel Automotive & Aerospace coatings India Pvt Ltd., and management who allowed us to use instrumentation, which is highly appreciated.
References
- Bentley, J.; Turner, G.P.A. Introduction to Paint Chemistry and Principles of Paint Technology. ISBN 0412723204. 1997.
- Talbert, R. (2007) Paint Technology Handbook. ISBN 1574447033.
- Woodbridge, P. R. (Editor) Principles of Paint Formulation. ISBN 0412029510, 1991.
- Deer, W.A; Howie; Zussman, J. (1992) An Introduction of Rock-Forming Minerals (2nd edition), Harlow, Longman ISBN_0-582-30094-0.
- American Society for Testing and Materials. ASTM D 3359 Standard Test Method for Measuring Adhesion by Tape Test; Philadelphia, PA, 1998.
- The mineral kaolinite - mineral galleries.
- Weismantel, G.E. Paint Handbook.
- Kapole, S.A.; Kulkarni, R.D.; Sonawane, S.H. Performance properties of acrylic and acrylic polyol-polyurethane-based hybrid system via addition of nano-CaCO3 and Nanoclay. Article first published online: Feb. 9, 2011.
- Maji, P.K.; Guchhait, P.K.; Bhowmick, A.K. Effect of Nanoclays on Physico-Mechanical Properties and Adhesion of Polyester-Based Polyurethane Nanocomposites: Structure–Property Correlations, Journal of Materials ScienceVolume 44, Number 21, 5861-5871.
- Garcia-Lopez, D.; Gobernado-Mitre, I.; Fernandez, J.F.; Merino, J.C.; Pastor, J.M. Properties of Polyamide 6/Clay Nanocomposites Processed by Low-Cost Bentonite and Different Organic Modifiers, Polymer BulletinVolume 62, Number 6, 791-800.
- Sabzi, M.; Mirabedini, S.M.; Zohuriaan-Mehr, J.; Atai, M. Surface Modification of TiO2 Nano-particles with Silane Coupling Agent and Investigation of its Effect on the Properties of Polyurethane Composite Coating, Progress in Organic CoatingsVolume 65, Issue 2, June 2009.
- Parvinzadeha, M.; Moradianb, S.; Rashidic, A.; Yazdanshenasc, M.E. Effect of the Addition of Modified Nanoclays on the Surface Properties of the Resultant Polyethylene Terephthalate/Clay Nanocomposites; Polymer-Plastics Technology and Engineering, Volume 49, Issue 9, 2010, Pages 874–884.
- Sung, L.P.; Comer, J.; Forster, A.M.; Hu, H.; Floryancic, B.; Brickweg, L.; Fernando, R.H. Impact of Nanoparticles on the Scratch Behavior of a Polyurethane Coating.
- Wypych, G. Handbook on Solvents, William Andrew Publishing, 2001.
- Wypych, G. Handbook on Filler. William Andrew Publishing, 1999.
- Mezger, T.G. The Rheology Handbook, published February 2011 ISBN: 9783866308640.
- Westhues, U.M. Polyurethane Coatings, Adhesive and Sealants, November 2007.
- Muller, B. Understanding Additives, January 1970 ISBN: 9783866308688.
- Poth, U. Automotive Coatings Formulation, May 2008 ISBN: 9783866309043.
- Schulz, U. Accelerated Testing, November 2008, ISBN: 978386630908.
- U. Poth, U.; Schwalm, R.; Schwartz, M. Acrylic Resins, published August 2011, ISBN: 9783866308572.
- Gysau, D. Fillers for Paints, published March 2011, ISBN: 9783866308701.
- Sander, J. Anticorrosive Coatings, published July 2010 ISBN: 9783866309111.
- Mischke, P. Film Formation in Modern Paints, published December 2009 ISBN: 9783866308619.
- Oyarzún, J.M. Pigment Processing, published May 2000, ISBN: 9783878705567.
- Dietrich, R. Paint Analysis, published August 2009, ISBN: 9783866309128.
- Bhushan, B. (Ed.), Springer Handbook of Nanotechnology. 2nd revised and extended edition. Springer Science + Business Media, Inc. Berlin, Heidelberg, New York 2007.
- Dosch, H.; Van de Voorde, M.H. (Eds) Gennesys. White Paper. Grand European Initiative on Nanoscience and Nanotechnology using Neutron – and Synchrotron Radiation Sources. Max-Planck-Institut für Metallfor-schung, Stuttgart 2009; www.mpi-stuttgart-mpg.de (Acces-sed 26 April 2010).
- Comyn, J. Adhesion Science, Royal Society of Chemistry Paperbacks, 1997.
- Kinloch, A.J. Adhesion and Adhesives: Science and Technology, Chapman and Hall, 1987.
- Israelachvili, J.N. Intermolecular and Surface Forces, Academic Press, New York, 1985.
- Newby, B.Z.; Chaudhury, M.K.; Brown, H.R. Macroscopic Evidence of the Effect of Interfacial Slippage on Adhesion, Science,1995, 269 (5229): 1407–9.
- Majmuder, A.; Ghatak, A.; Sharma, A. Microfluidic Adhesion Induced by Subsurface Microstructures. Science,2007, 318 (5848): 258–61.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!














