Reducing VOCs from Surfactants in Coatings

















The original limit was 250 g/L for a flat architectural paint, but this was reduced in 2008 to 50 g/L.2 VOCs can improve coalescence, flow and leveling, open time, and even freeze-thaw stability. In previous VOC limit reductions the industry was able to reduce VOCs without much impact on the formulation; however, the new 50 g/L limit has caused coatings manufacturers to re-examine their formulations and look at new ways to reduce VOCs. In some cases, manufacturers had to re-invent their coatings to meet the new standard.
To compound the issue, the SCAQMD also changed colorant VOC limits. The new limit, effective January 1, 2014, is also 50 g/L.2 Many colorants utilize large amounts of VOCs to allow ready dispersibility into the coating when mixed. The VOCs include solvents and glycols. Similar to the 50 g/L VOC limitation on paint, manufacturers have had difficulty meeting the new standard on colorants. This has even led to the use of alternative pigments that can more easily be dispersed without large amounts of VOCs. Already, many suppliers are advertising zero-VOC colorants for architectural tinting systems.3
In addition to changing VOC regulations, the method for measuring VOCs has also been in flux in the recent decade. The long-standing method for VOC measurement was EPA Method 24. This is a fairly harsh test in which a coating sample is weighed and heated in an oven at 110 °C for 1 h. The sample is then re-weighed. The difference, after subtracting the water and VOC-exempt solvents, is the VOC content.4 Many issues arose from this test. One was the possibility of getting negative numbers. The sample could absorb moisture from the atmosphere and, after heating, the material could actually have a negative VOC content because of the loss of this moisture. Additionally, oven size can impact the test results.5 Also, the test allowed for greater error at low VOC content. To help better define what a VOC is and to avoid using Method 24, the European Union adopted a definition for VOC in 2002, which states a VOC is “any organic compound having an initial boiling point less than or equal to 250 °C measured at a standard pressure of 101.3 kPa.”6 This shifted the testing from the coating formulation to individual components initially. A gas chromatography (GC) test using a boiling point column was developed to support this method, which could test the entire coating. In the United States, various regulatory agencies considered developing a new method. This melded around a new ASTM Method D6886. Again, a GC is utilized with reference standards to determine the VOC content of a coating.7
Traditionally, the surfactants utilized in paints and colorants were based on alkylphenol ethoxylates (APEOs). Due to the unique branched structure, the surfactants provided good pigment and substrate wetting while maintaining a low pour point. In the alkoxylation, a typical aqueous caustic catalyst is added for the EO addition, and water is removed to dehydrate the catalyzed mixture and to form the phenoxide anion. As the phenol group is slightly more acidic than the hydroxyl group on a primary alcohol, the phenoxide anion is easier to form than the alkoxide anion. This leads to a more narrow-range EO adduct distribution compared to base-catalyzed primary alcohol ethoxylates. Additionally, it significantly reduces the levels of free un-ethoxylated phenol, which means the VOCs are much lower for APEOs. Two common APEOs are octylphenol ethoxylates and nonylphenol ethoxylates (Figure 1). These surfactants would be excellent to aid in the reduction of VOCs in coatings and colorants, but due to governmental regulations and end-use customer demands, APEOs are not being utilized as the coating and colorant formulations are re-formulated. Therefore, the coatings industry is seeking alternative surfactants not based on APEOs.
Narrow-Range Ethoxylation
Alternatives to APEOs can be a difficult proposition due to the ability to match the physical properties for many ethoxylates. These include properties such as similar gel points, color and low free alcohol content, which are not easily achieved. This is especially true when linear alcohols are ethoxylated with potassium hydroxide, the typical catalyst for ethoxylation. For this reason, branched hydrophobes and narrow-range ethoxylation (NRE) catalysts are employed. The combination has shown significant improvement in generating products similar to APEOs.
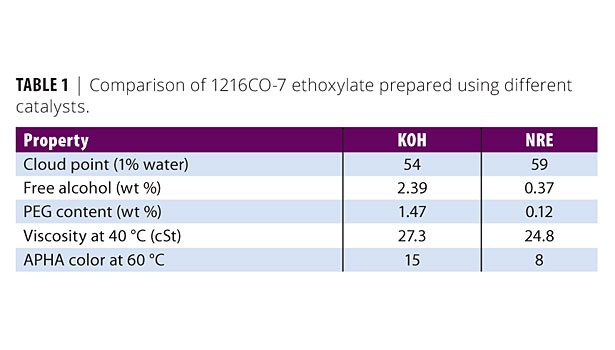
Typically, the distribution of ethoxylation oligomers (“ethoxymers”) seen in an APEO is narrower than that of an alcohol ethoxylate catalyzed with potassium or sodium hydroxide (KOH and NaOH, respectively). However, when NRE catalysts are used, the ethoxymer distribution better mimics that of the APEO. Figure 2 illustrates the change in ethoxymer distribution when using conventional catalysts (NaOH and KOH) compared to the NRE catalyst. The product manufactured is a linear C12 to C16 alcohol 7 mole ethoxylate (1216CO-7).8 The differences between the resulting distributions play an important role in the subsequent physical properties of the ethoxylate (Table 1). In many cases, these small differences will also determine if the ethoxylate can be used to replace an APEO or not. For example, the alcohol ethoxylate produced with the NRE catalyst exhibits less free alcohol content, lower polyethylene glycol (PEG) levels and better color for high mole products in comparison to the ethoxylates produced with conventional catalysts.8-9 The free alcohol and the PEG level would contribute the largest percentage to the VOC content; therefore, minimizing these is critical to reducing the VOC content of the final nonionic surfactant.
Previous APEO Alternatives
In prior work, suitable alternatives to APEOs were found for coatings applications. These were ethoxylates based on isotridecyl alcohol (TDA) based on n-butene feedstock and Fischer-Tropsch (“FT”) process-based alcohols (FT-OXO) with a carbon range of 12 to 13 (Figure 3).10
These ethoxylates were produced with NRE catalyst. The surfactants presented physical properties that were very close to those of the APEOs. In addition, the coating and colorant properties were equal to or better than the standard APEOs, but the VOC implications were not examined in that study.11 To better assess these repercussions, this study was initiated using both the current Method 24 test protocol and the new ASTM 6886 VOC test method. The following sections discuss the surfactants evaluated and the VOC testing of these materials.
Experimental
Twelve nonionic surfactants were examined including two industrial standards: an octylphenol ethoxylate (OPE-10.5) and a nonylphenol ethoxylate (NPE-9). The remaining samples were ethoxylates based on conventional (KOH) and narrow-range (NR) ethoxylation technology with n-butene-based isotridecyl alcohol (referred to as TDA-X with X being the number of EO moles) and a FT-OXO alcohol (referred to as 23-X with X being the number of EO moles) as the starting hydrophobes (Table 2).
Testing Methods
EPA Method 24 was followed using the procedure outline by the EPA.4 A Karl-Fischer titration was utilized to determine the water content of each ethoxylate. The sample ethoxylates exhibited essentially no water present, as expected. The water, although very low, was utilized in the calculations.
ASTM D6886 was followed as outlined in the procedure.7 The reference marker was methyl palmitate, which is the VOC cutoff marker designated by the SCAQMD. The samples were run in triplicate and averaged.
Results and Discussion
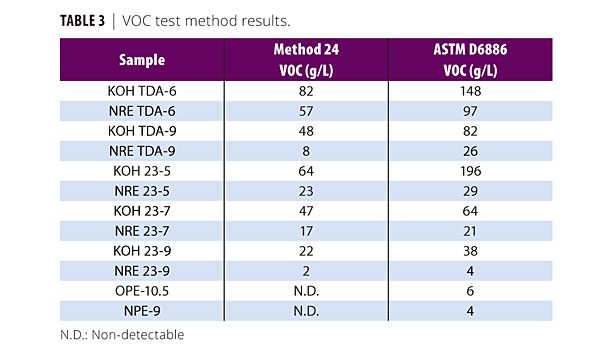
The results of the test methods can be seen in Table 3. Clearly, differences in the measured VOCs are evident. With each product, Method 24 results in lower VOCs compared to those obtained by ASTM D6886. The -latter method appears to pick up on smaller amounts of VOC present. This may be attributed to the greater sensitivity of the GC method as opposed to Method 24’s gravimetric analysis. Despite this, the two methods do demonstrate similar trends between the various ethoxylates. It should be noted that each method was designed for examination of coatings, not to specifically look at individual formulation components.
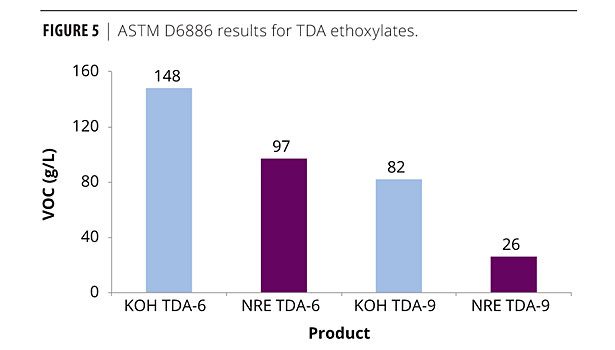
Upon examining the classes of materials, NR ethoxylates, as expected, differed from their conventional analogs. Figures 4 and 5 outline the TDA series. Increasing the number of moles of EO on the ethoxylate is already known to reduce the free alcohol present in the finished product,11 therefore, a VOC reduction from TDA-6 to TDA-9 was expected. The NR ethoxylation further reduced the amount of VOCs - in some cases by more than 50% versus the ethoxylates based on the conventional catalyst.
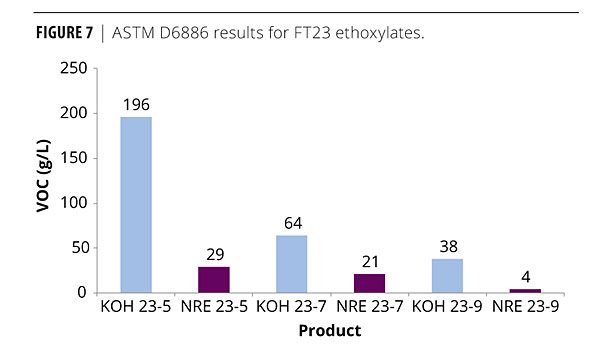
Focusing on the FT 23 alcohol, similar trends to the TDA series are visible (Figures 6 and 7). As the moles of EO increase from 5 to 9, the VOCs drop from 196 to 38 for the conventional ETO catalyst (ASTM D6886). The NR ethoxylation also experienced a drop as the moles of EO increased. Interestingly, NR 23-5 already has lower VOCs measured by either method than the conventionally catalyzed 23-9. Another observation is that the FT 23 NR ethoxylates have lower VOCs that the TDA NR ethoxylates. This relates to the structure of the starting alcohol. Less branching at the C2 position for the FT 23 alcohol allows for easier addition of EO when compared to TDA; therefore, more of the alcohol present is converted into ethoxylate, leaving less free alcohol available to be accounted as a VOC.
It should be noted that the NR ethoxylation catalyst is known to reduce the PEG in the finished ethoxylate, as well. As this PEG will also be a distribution, low mole PEGs may be evident as a VOC in either test method. This would increase the overall VOC content and would be more pronounced in samples with higher PEG, i.e., the conventionally catalyzed ethoxylates. Further GC-MS work could be utilized to demonstrate this idea but was outside the scope of the presented research.
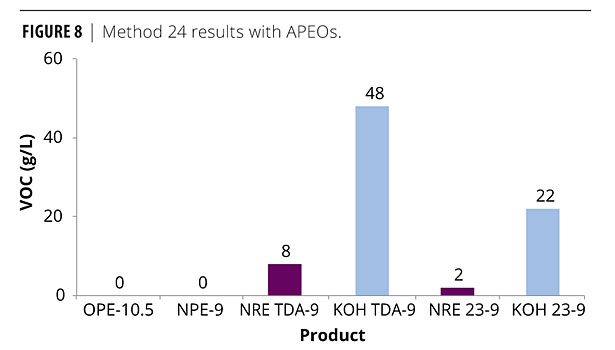
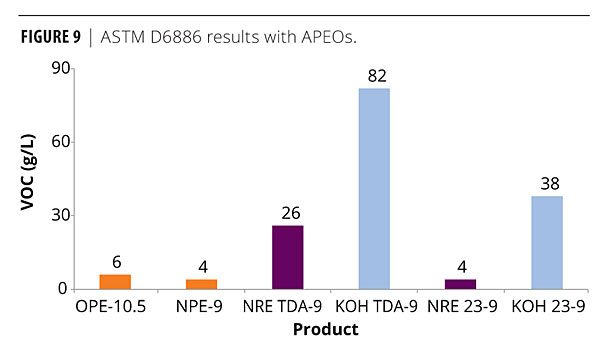
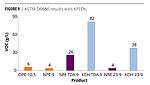
Finally, Figures 8 and 9 show a comparison with the APEO standards OPE-10.5 and NPE-9 with the 9 mole TDA ethoxylates and the FT 23 alcohol ethoxylates. The APEOs actually gave negative VOC numbers for Method 24, which is illustrated in the graph as zero. As mentioned earlier, this is usually due to the absorption of atmospheric moisture. Using ASTM D6886, the two APEOs show very low VOCs < 10 g/L. Only the NR 23-9 gives a similar response. However, compared to the conventional TDA-9, the NR TDA-9 is much closer to the VOC content of the APEOs.
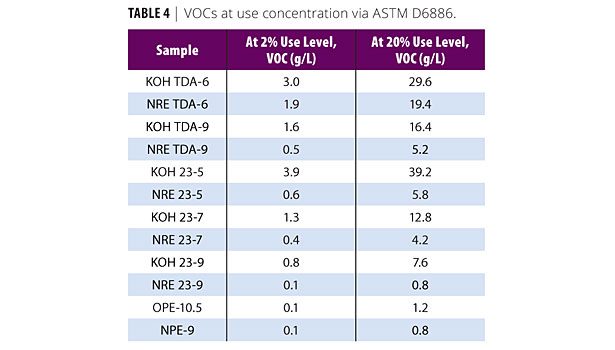
Again, both VOC tests were intended to be used on a finished coating. In this study, only the pure nonionic surfactants were examined, but if the surfactants were tested in formulations at typical use levels, the VOC contribution of the surfactants would be much lower. Knowing the contribution of the surfactant is critical in determining which surfactant to use, because with VOC requirements as low as 50 g/L, every part of the formulation must be inspected to find areas to reduce the VOC content. To simulate the contribution of the surfactant to the overall VOCs of a coating formulation, the VOC content of the pure surfactants using ASTM D6886 was multiplied by the typical loading level. In coatings this would be 2%, and for colorants this would be about 20% (Table 4).
Examining this data, the conventional surfactants, KOH TDA-6 and KOH 23-5, constitute 6% and 6.5% of the allowable 50 g/L of VOC at 2% loading level. This leaves less formulation space for other materials such as co-solvents, coalescents, freeze-thaw stabilizers, etc., when compared to the NRE analogs that yielded only 3.8% for TDA-6 and 1.2% 23-5, respectively. A similar analogy can be made from the 20% surfactant loading level; the conventional surfactant makes up to ~78.4% of the 50 g/L limit. Clearly, NR ethoxylates reduce the VOCs under any condition by more than half.
Table 4 also illustrates the very low contribution to the VOC content from the industrial standards OPE-10.5 and NPE-9. Again, despite the low VOC, the APEOs are still being phased out in most formulations, but the NR surfactants such as NR TDA-9, NR 23-7, and NR 23-9 exhibit VOC contributions that mimic the APEOS and would, thus, be viable low-VOC alternatives.
Conclusions
Government regulation and customer preference have driven the coatings industry to reduce VOCs. With limits approaching zero VOC, every compound in the coatings formulation is under scrutiny for its VOC content. In the area of surfactants, this is further complicated by phasing out low-VOC-containing APEO surfactants. Therefore, alternatives are needed to meet the APEO-free requirement while maintaining a low-VOC content. The use of NR ethoxy-lation technology and branched hydrophobes allows the alternatives not only to match the properties of the APEOS as reported in a previous paper but also the VOC content as demonstrated in this article. Despite change in the method for measuring the VOC content, the NR ethoxylates remain a viable low-VOC APEO alternative. n
Acknowledgements
We would like to acknowledge The University of Southern Mississippi and California Polytechnic State University, San Luis Obispo, for help in evaluating the surfactants.
This paper was presented at the 39th Annual Waterborne Symposium in New Orleans.
References
1 EPA’s National Volatile Organic Compound Emission Standards for Architectural Coatings, http://www.epa.gov/ttn/atw/183e/aim/aimpg.html.
2 AQMD, Rule 1113, Table of Standards, http://www.aqmd.gov/prdas/coatings/table_of_standards.htm, retrieved on 12/1/11.
3 Phillips, D. No-VOC Colorants for Architectural Tinting Systems, PCI Magazine, June 1, 2011.
4 EPA, Method 24 - Determination of Volatile Matter Content, Water Content, Density, Volume Solids, and Weight Solids of Surface Coatings, http://www.epa.gov/ttn/emc/promgate/m-24.pdf, retrieved on 12/1/11.
5 Jones, D. R., et. al. Development of an Improved VOC Analysis Method for Architectural Coatings, http://www.arb.ca.gov/research/seminars/jones2/jones.pdf, retrieved on 12/1/11.
6 Directive 2004/42/CE of the European Parliament and of the Council of 21 April 2004 on the Limitation of Emissions of Volatile Organic Compounds Due to the Use of Organic Solvents in Certain Paints and Varnishes and Vehicle Refinishing Products, EUR-Lex; European Union Publications Office; http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32004L0042:EN:NOT ; retrieved on 12/1/11.
7 ASTM D6886.
8 Matheson, K. L., et. al., Process for Preparing Alkoxylation Catalyst and Alkoxylation Process, US2007213554, (2007).
9 Sharp, M. A., et. al., Developments in Alcohol Ethoxylation Technology, 98th AOCS Annual Meeting and Expo, (2007).
10 Sharp, K.;Sharp, M.;Matheson, K.L. Alkylphenol Ethoxylate Replacement in Emulsion Polymerization 35th Waterborne Symposium Proceedings, 2008.
11 Sharp, K.; Sharp, M.; Matheson, K.L. Alkylphenol Ethoxylate Replacement in Coatings 36th Waterborne Symposium Proceedings, 2009.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





















