New Antimicrobial Coating
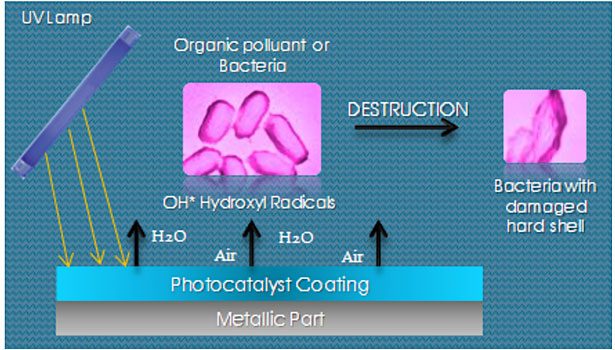





Photocatalysis is a natural phenomenon in which a photocatalyst accelerates the speed of a chemical reaction under the action of light (natural or artificial). Using light energy, the photocatalyst generates the transformation of water and oxygen from the ambient air, generating highly reactive molecules called free radicals, which are able to break down organic substances (sometimes harmful), into completely harmless compounds by a redox reaction.
TiO2 is a semiconductor material; electrons in it are therefore able to change their energy state. If we give them enough energy they will move from the valence band to the conduction band across an energy gap called "band gap" (theory of band /solid state physics). This change of state generates a "hole" − a deficit of electrons in the valence band that will be called "h+", and an excess electron in the conduction layer, which will be called "e-".
Holes "h+" react with water to form hydroxyl radicals OH°, and the electrons react with oxygen to form superoxide 02-. These are two powerful oxidants that can destroy organic molecules such as bacteria, viruses, germs, odors, volatile organic compounds, mold, algae, fungi, pollen, dust mites, etc.
The Basic Reactions
TiO2 + UV èh+ + e- pair creation: hole/electron
H2O + h+ è°OH + H+ oxidation of water, hydroxyl formation
O2 + e- èO2- reduction of oxygen, superoxide formation
Energy can be supplied in several ways. In our case it is provided by light radiation. This light radiation needs to be higher than the band gap’s energy so that the change of state can happen. Condition: Light energy > 3.2 eV (electron volt) minimum, which corresponds to light of wavelength shorter than 388 nm è UV-A.
Our TiO2 has been optimized to reduce the value of "band gap" energy; therefore it is also active with visible light.
Effectiveness of the Layer
The photocatalytic ability of SaniUV is characterized by measuring an efficiency coefficient called the Catalytic Improvement Factor (CIF). This coefficient corresponds to a specific degradation rate of methylene blue (MB), similar to the one mentioned in ISO 10678.(1) It involves measuring the difference in concentration of a solution of MB in contact with a coated surface before and after 24 h exposure under UV-A.
To date, SaniUV has a CIF of 9.82 on a scale from 0 to 10, which corresponds to a complete discoloration of the solution of MB within 24 h, clearly showing its high photocatalytic activity.
In addition, SaniUV has a CIF of 2.5 when it is exposed to visible light. For comparison, coated windows of building have a CIF of 1.8 under UV-A. Because of its crystalline structure, the effectiveness of the layer is proportional to its thickness.
The photocatalytic ability of the coating therefore gives an antimicrobial effect. The antimicrobial ability, characterized by the ISO 27447,(2) consist of comparing the evolution of two cultured E. Coli populations under UV, one on a coated surface, the other on a neutral surface.
This method measures a factor called "Log Reduction" corresponding to the number of decades in which the initial population of bacteria was divided 8 h after UV exposure. For example, a surface capable of dividing the amount of bacteria by 10,000 (104) in 8 h has a "Log Reduction" of 4. There is another unit of measurement known as "decimal reduction time," being the time necessary for a surface to divide a population of bacteria by 10
The French pharmacopoeia speaks of sterilization when it remains one bacteria on a million present before the sterilization operation, so sterilization is obtained with a reduction of bacteria in the order of 106 (Log Reduction = 6) .
To date, we have not measured accurately the "Log Reduction" of our SaniUV coating. However, the antimicrobial effect is directly related to the photocatalytic effect. Our CIF of 9.82 suggests a particularly high antimicrobial potency.
Enabling SaniUV
Through optimization of the coating, the photocatalytic reaction is now possible with visible light, and SaniUV is active with the majority of lighting (neon lights, compact fluorescent bulbs, LED lamp). However, for optimal effectiveness it is best to expose the layer to a light having a wavelength shorter than 388 nm (UV-A, UV-B, UV-C). The shorter the wavelength, the more swift the reaction will be.
The coating has a memory effect. In fact, after UV exposure the coating remains active for a while, preventing the proliferation of new contaminants.
SaniUV is biocompatible and can be used on medical devices, from a simple surgical instrument to a medical endoscope. With a CIF of 9.82, SaniUV distinctly demonstrate its industrial advance ahead others photocatalytic technologies.
References
1 ISO 10678: Fine ceramics − Determination of photocatalytic activity of surfaces in aqueous medium by degradation of methylene blue.
2 ISO 27447: Fine ceramics − Test method for antibacterial activity of semiconducting photocatalytic materials.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!










