VOC-Free Alternative for Green Coatings: The Future of Emulsion Polymerization





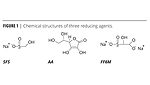






Emulsion polymerization redox systems have historically used ascorbic acid (AA) or sodium formaldehyde sulfoxylate (SFS) reducers along with t-butyl hydroperoxide (TBHP) oxidizer. These systems are well established and have been used for emulsion polymer production for more than 50 years. Bruggolite FF6 M is relatively new, is more powerful than either AA or SFS, is formaldehyde free and is best suited for lower-pH reactions.
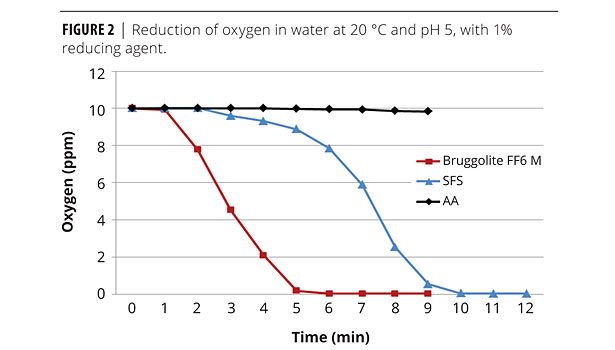
These three reducing agents are represented in Figure 1. Their comparative effectiveness as reducing agents can be seen in Figure 2, where SFS takes twice as long to remove oxygen as FF6M. Both are significantly more effective than AA.
Example Polymerization Data
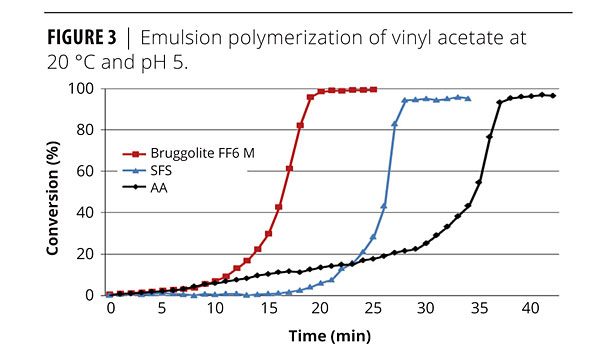
A model emulsion polymerization of vinyl acetate to PVAC has been thoroughly studied and demonstrates the effectiveness of FF6 compared to SFS and AA when used as the main polymerization reducing agent. This system clearly shows higher conversion percentage and shorter conversion time for an FF6 redox system (Figure 3).
Similar results can be seen when FF6 is used as the reducing agent for vinyl acetate and VeoVa™ systems, and substituting H2O2 for TBHP, resulting in a VOC-free process (Figure 4).
In a test polymerization with another vinyl acetate system, the aim of the evaluation was to reduce the residual monomer content of vinyl acetate in two given latices with the help of FF6 down to a level below 1000 ppm using VOC-free techniques. TBHP was excluded; the oxidizer was H2O2. The target time for post polymerization was 60 min. Figure 5 depicts the rapid monomer conversion and very low residual monomer levels achieved, exceeding the expectations of the formulation in both time and residual monomer level.
Recommended Process Conditions
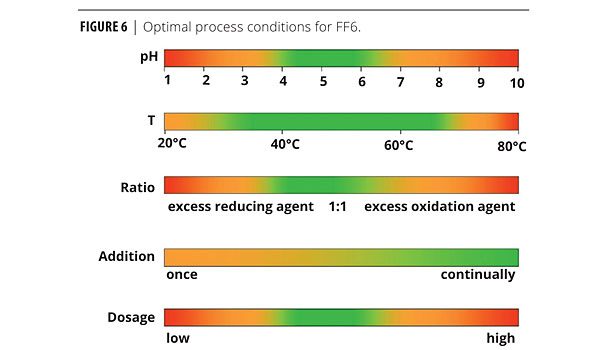
For optimized monomer conversion and reaction time, FF6 works best in the range of process conditions depicted by the green ranges shown in Figure 6.
By its chemical nature, FF6 shifts more to the acid side of the salt/acid balance at lower pH values. Very low-pH emulsion systems may result in rapid generation of free radicals and reaction termination. A similar result can be seen at higher temperatures. Where thermal splitting of SFS can begin at 90 °C, FF6 will begin to split at 65 °C. The goals for pH and temperature settings are to manage the generation of free radicals to control the polymerization reaction at a steady pace.
The ratio of FF6 to oxidizer and addition rate should also be controlled to optimize the reaction. Best performance has been observed when these redox agents are added continuously on a 1:1 ratio.
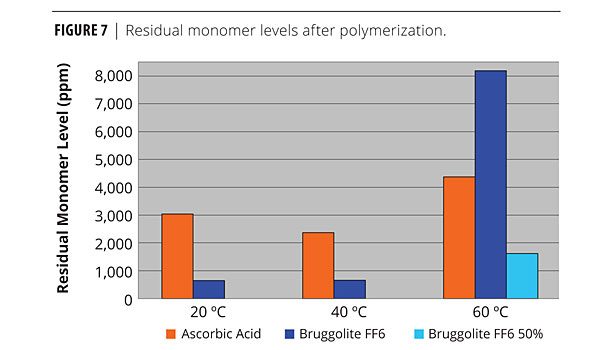
It has been observed that less FF6 is needed for effective emulsion reactions versus SFS. This is due to the higher reactivity of FF6. It is believed that excess dosage of FF6 can destroy free radicals and terminate polymerization, as can be seen in Figure 7. This clearly shows that when less FF6 is used, it will result in lower residual monomer content at the end of the polymerization, especially at elevated temperatures.
Measuring and Controlling Redox Potential
Recent developments in redox potential measurement probes now enable better control of the addition rate of redox chemicals and provide insight into reaction time optimization. As can be seen in Figure 8, the redox potential (red line) goes from negative to positive as the polymerization progresses. In this model reaction, the redox addition rate is controlled to maintain 60 °C tank temperature, providing an overall uniform, fast polymerization rate. As the redox potential reaches positive, it indicates the polymerization has essentially reached completion. Further reactor time is unnecessary, demonstrating that redox potential monitoring can improve productivity.
Polymer Yellowing
Tests have shown that FF6 will reduce polymer yellowing and oxidation, both during and after polymerization. It has also been shown that FF6 can provide acceptable reaction rates without the addition of iron, which further contributes to polymer color retention (Figure 9).
Conclusions
When used in the main polymerization step, more controlled reactions are possible with an FF6 redox system. Polymers can be tailor made with improved physical properties. Slow, controlled initiation of the polymerization is possible with no retardation later in the reaction cycle. Lower-temperature initiation for free radical generation is also possible, avoiding side reactions and crosslinking. This can result in tighter control of molecular weight distribution.
When used in the post polymerization step, physical stripping of residual monomers may not be necessary, resulting in less energy consumption. Recycling of monomer and associated process equipment may not be necessary.
For both main and post polymerization use, FF6 can provide faster reactor throughput and more complete conversion of monomer to higher-quality polymer. But perhaps the biggest advantage is that low-VOC polymers can be made with formaldehyde-free redox agents.
VeoVa™ vinyl esters of Versatic™ acid are a product of Momentive Specialty Chemicals Inc., Columbus, OH.
For more information, visit www.momentive.com/veova.
Bruggolite® FF6 M is a product of Brüggemann Chemical, Industrial Chemicals, Newtown Square, PA.
For more information, visit www.brueggemann.com/english/chemical-us.html.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!