Troubleshooting High-Performance Epoxy Systems












• Raw material selection (resins, modifiers, curatives, pigments, additives, solvents, etc.);
• Stoichiometry and mix ratios;
• Cure chemistry and catalysis;
• Rheology;
• Film formation;
• Adhesion; and
• Corrosion.
Good epoxy formulators clearly understand these factors. These are their stock-in-trade, and they would be incapable of developing high-performance formulations without properly understanding these issues. However, experience has shown that many details about epoxy resins are generally not well understood, even by the best formulators. These details may be viewed as minutia, relevant only to the epoxy resin chemist. Yet, experience has shown that these details can cause performance failures or other problems in high-performance systems. If these details are not understood, then performance problems may be misdiagnosed, and, as a result, may not be properly solved.
Bisphenol A Epoxy Resins
The focus here is on bisphenol A (BPA) diglycidyl ethers, which constitute well over 97% of all epoxy functional materials produced in the world. BPA resins are used in virtually all epoxy coatings. They are versatile building blocks that are used in a broad spectrum of high-performance coatings, including:
• Aerospace primers;
• Automotive primers (cathodic electrodeposition);
• Beverage and food can linings;
• Chemical-resistant tank and drum linings;
• Industrial maintenance coatings (factories, refineries and chemical plants);
• Marine coatings (ships and offshore structures);
• Pipe coatings (oil and gas transmission); and
• Transportation coatings (railroad, trucks, buses).
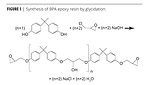
BPA epoxy resins are made by reacting bisphenol A with epichlorohydrin in a reaction termed “glycidation”, as outlined in Figure 1. A BPA epoxy resin like that in Figure 1 with a repeat unit “n” very near to zero can be obtained, and is available commercially. It has an epoxy equivalent weight (EEW) of about 172-176, and a viscosity of about 40-60 Poise.
The Liquid is a Solid!
The BPA epoxy resin just described (n ≈0) is actually a crystalline solid at room temperature, with a melting point of about 42 °C. (Similarly, low-viscosity bisphenol F epoxy resins can crystallize in a like manner. The melting point of these crystals is about 80-85 °C.) In its liquid state, it is a super-cooled liquid at room temperature. As such, the solidification is unpredictable. It might happen in three days, three months or three years. All that we know for sure is that the thermodynamically stable form is a crystalline solid. Evidence of crystallization in a BPA epoxy resin ranges from haziness or cloudiness in the resin to a slushy mix of liquid and solid, to near complete solidification. Crystallization occurs in the temperature range between the glass transition temperature of the resin (in the range -20 to -10 °C) to the melting point (42 °C). Storage at cool temperatures or cycling the temperature seems to increase the likelihood of crystallization. Crystallization is reversible, and a crystallized sample can be warmed above the melting point of 42 °C to melt the crystals and return the sample to a crystal-free condition.
Crystallization can cause “seeds” in epoxy coatings. This is insidious, because the coating can pass quality control testing with no crystals present. However, they can form at a later time, resulting in an unacceptable, seedy coating.
Lowering the viscosity of the resin generally increases the propensity to crystallize. This includes adding reactive diluents and solvents, which is commonly done in formulating coatings. It is believed that lowering the viscosity increases the mobility of the resin molecules, enhancing their ability to form crystals.
Pigment and filler particles can serve as nucleation sites for crystallization. Obviously, pigments are also important components of epoxy coatings.
Epoxy resins with lower EEW have greater propensity to crystallize than do higher-EEW liquid resins. It is believed that the higher-molecular-weight oligomers in the higher-EEW resins act to disrupt the crystal structures and prevent crystallization. Table 1 summarizes the characteristics of four commercial liquid BPA epoxy resins. The resin with an average repeat unit, “n”, of about 0.12 has good crystallization resistance with reasonably low viscosity. This has been established as the standard, general-purpose liquid epoxy resin by virtually every epoxy manufacturer in the world. The two lower-viscosity resins and the higher-viscosity resin are specialty products that are used in applications where less resistance to crystallization or higher viscosity can be tolerated.
Crystallization Example
A company was formulating high solids, low-VOC, two-pack epoxy coatings. Originally, these coatings were formulated with the “standard” liquid epoxy (n ≈0.12). A formulator realized that they could achieve lower viscosity and lower VOC by shifting to one of the lower-viscosity liquid resins. The reformulation was done, however the formulator was not aware of this crystallization issue. They were successful with this reformulation for some time, but then encountered a batch that developed “seeds.” These seeds were identified as epoxy crystals. This seedy batch had been filled into quarts, gallons and five-gallon pails, and was in distribution channels. It had to be recalled. Reprocessing could potentially eliminate the crystals, but recovery from many small packages would be problematic and expensive. Failure to understand this “subtle detail” about epoxy resins resulted in an expensive failure.
Non-Epoxy End Groups
The reaction in Figure 1 is an idealized scheme that ignores all potential side reactions, therefore the structure of the epoxy resin shown is also idealized. This structure shows a molecule with two epoxy end groups. The material is difunctional (in epoxy). This is one of the most prominent misconceptions about epoxy resins. In fact, in a liquid BPA epoxy resin, 99+% of the end groups are epoxy, as shown. So less than 1% are non-epoxy. One may easily conclude that discussion of less than 1% of end groups is truly a subtle detail that is of interest only to epoxy resin chemists. But these non-epoxy end groups can have significant effects, which should not be ignored. The following discussion will establish how understanding this is also quite important to the epoxy formulator
A liquid BPA epoxy resin is nearly difunctional. However, as one goes up in molecular weight, the number of epoxy groups decreases, while the number of non-epoxy end groups remains constant. Thus, in a high-molecular-weight resin, the relative amount of non-epoxy end groups can be significant. For example, in an epoxy resin with an epoxy equivalent weight of about 3000, the average epoxy functionality can be only about 1.3 or so. This is clearly not a difunctional material.
Examples of non-epoxy end groups are summarized in Figure 2. The phenolic end group results from incomplete glycidation of phenolic hydroxyls. The two chlorohydrins result from incomplete dehydrochlorination during resin processing. The chlorohydrins are commonly referred to as hydrolyzable chlorine. The first fixed chlorines result from an atypical reaction of BPA and epichlorohydrin to form a molecule that does not undergo dehydrochlorination. The second fixed chlorine is formed by the glycidation of a chlorohydrin. The alpha glycol results from hydrolysis of epoxy groups during resin processing. (The principal mechanism involves conversion of epichlorohydrin glycidol. Glycidol reacts with phenolic OH to give the alpha glycol shown.)
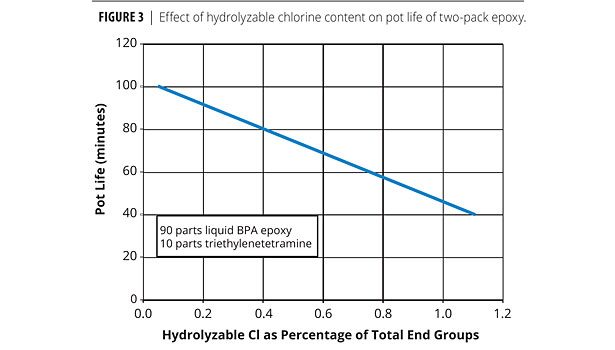
The phenolic, chlorohydrin and alpha glycol end groups serve as accelerators in the cure of epoxy resins with amines or amine-functional curing agents. The relative levels of these end groups can influence the gel times of some coating formulations. Figure 3 shows the acceleration effect (decreasing pot life) of hydrolyzable chlorine on amine-cured epoxy. This demonstrates how even small and subtle changes in end groups can have significant impact on performance.
Epoxy equivalent weight is often interpreted as an approximate measure of molecular weight. This interpretation is based on the belief that all epoxy resins are difunctional in epoxy groups. Increasing the amount of non-epoxy end groups will then increase this apparent molecular weight. In systems cured through epoxy groups, non-epoxy end groups do not participate in crosslinking and have the effect of decreasing the crosslink density of the cured epoxy matrix. Increasing non-epoxy end groups at a given EEW decreases resin molecular weight and viscosity.
Some epoxy resin manufacturers may purposely introduce non-epoxy end groups, or may manipulate them to modify resin properties, such as lowering viscosity or increasing reactivity.
BPA epoxy resins are generally made by two possible routes.1 In the taffy process, BPA and epichlorohydrin are reacted together at appropriate ratios to make epoxy resins of any desired molecular weight. In the fusion process, BPA and liquid BPA epoxy resin are reacted together at appropriate ratios to make epoxy resins of any desired molecular weight. Taffy resins appear to give lower viscosities than equivalent fusion resins because taffy resins generally have higher levels of non-epoxy end groups than do fusion resins (Figure 4). So at a given molecular weight, the taffy resin will have slightly lower epoxy content and higher epoxy equivalent weight. The higher EEW will make it appear to be a higher-molecular-weight resin than it truly is. It will have the viscosity of a resin of its molecular weight, but it will appear to be a higher-molecular-weight resin with an anomalously low viscosity.
End Group Manipulation
This principle has been exploited to make resins with apparently low viscosity. To do this, epoxy resins are made with monofunctional chain terminators that serve as non-epoxy end groups. To illustrate this, assume we make epoxy resins by reacting BPA, liquid epoxy resin (EEW = 188) and small amounts of phenol in ratios to achieve an EEW of about 3000. Figure 5 summarizes what we would expect to find. The phenol serves as a chain terminator and causes the number average molecular weight (Mn) to decrease as the phenol is increased. Notice, however, that the viscosity drops even faster than the molecular weight. This is an illustrative example only. Phenol may not be actually used. However, other monofunctional chain terminators are used to lower viscosity, increase reactivity or both.
More subtle effects may be encountered. One example is when the reaction between liquid epoxy resin and BPA stops before 100% completion (e.g., before 100% BPA consumption). This increases both the number of phenolic end groups and epoxy end groups, resulting in lower resin molecular weight and viscosity. To illustrate this, assume that we intend to make an epoxy resin with an EEW of about 3000. What kind of resin would we expect to get if the reaction is taken to completion? Or what would result from only 98% completion? Or what would result from 96% or 94% completion? Figure 6 summarizes these expected differences. It is clear that stopping the reaction only a few percent short of completion reduces molecular weight and EEW, but reduces viscosity even more dramatically.
All Epoxy Resins May Not Be Equal
Many people believe that all BPA epoxy resins are basically the same, regardless of manufacturer. It does not matter which one is used in a formulation that calls for this type of liquid epoxy. This is an assumption that is made based on the fact that all these resins have essentially the same epoxy equivalent weight and viscosity. But the non-epoxy end groups are ignored here, so the assumption may not be correct.
As an example, one epoxy manufacturer sells liquid BPA epoxy resin with one level of alpha glycol. However, another manufacturer sells an “equivalent” resin with 3+ times as much alpha glycol. Are these equivalent? If the coating formulation is insensitive to alpha glycol level, then they would appear to be equivalent. It is known that cathodic electrodeposition coatings may give better performance if the epoxy feedstocks are low in alpha glycol. In such a case, these resins would not be equivalent.
As another example, two general grades of liquid BPA epoxy resin are sold. One grade has a low level of hydrolyzable chlorine (low chlorohydrin content), while the other grade may have levels 3+ times higher. If a coating formulation is insensitive to hydrolyzable chlorine, then these grades would be equivalent. Under certain circumstances, the chlorohydrin could dehydrochlorinate and release inorganic chloride in the coating. This could be detrimental to corrosion resistance. As an example, cathodic electrodeposition coatings would generate this inorganic chloride during their manufacture. Resulting high levels of chloride would attack the anodes in the bath, requiring premature replacement. In one incident, a customer making cathodic electrodeposition resins inadvertently used a batch of epoxy resin with high hydrolyzable chlorine. This resulted in premature failure of the anodes in the baths where it was used. It also resulted in complaints and financial claims by the coater.
It may be more prudent to not make the assumption that all resins are equivalent. It is important to know what levels of non-epoxy end groups are in the resins used in your formulations, and how these end groups affect the performance of your high-performance coatings.
Epoxy Resin Suppliers
The non-epoxy end groups cannot be eliminated. They are a result of side reactions that occur during the manufacture of epoxy resins, as outlined in Figure 1. Epoxy resin manufacturers can only minimize them and control them within the constraints of the process and equipment that each manufacturer uses. Also, the levels of non-epoxy end groups are related. For example, if one tried to further minimize, or even eliminate the hydrolyzable chlorine (chlorohydrins), the side reaction forming alpha glycol would be strongly favored, and the alpha glycol content would go up dramatically. In the author’s view, the major epoxy manufacturers do an excellent job of minimizing and controlling these end groups to provide very consistent resins.
Liquid epoxy suppliers generally establish specifications for epoxy equivalent weight, alpha glycol content, hydrolyzable chlorine content, color and viscosity. They work hard to control these characteristics within their processes and to deliver product within specification. They generally do not establish specifications on such factors as total chlorine, phenolic hydroxyl content, functionality and molecular weight. They may establish typical ranges for characteristics like total chlorine and phenolic hydroxyl.
It is strongly recommended that formulators contact their suppliers and get the specifications and typical ranges for the epoxy resin characteristics they use. Certificates of analysis should be kept on file for all epoxy resins used in both product development and in manufacturing commercial products. This data can be invaluable in troubleshooting potential future problems.
Example: High-Performance Coating Failure
A coating manufacturer developed a unique high-performance coating that was carefully optimized to achieve the desired performance. The coating was evaluated, approved and specified by their customer. After this qualification, it was found that if any of the several factors described above varied – even within established typical ranges and specifications – the coating performance could go from excellent to failure. It was determined that the resin requirements needed to achieve necessary coating performance were so narrow, that the resin supplier had to lot-select resins from inventory for this application. As a result, the supplier could not guarantee resin availability. There was no way to ensure that resins with the very narrow requirements would be available at all times. Specifications are normally set around process capabilities. If a customer needs a product with tighter specifications then, by definition, this requirement is beyond the process capability, and the process is incapable of generating that required product at all times.
Furthermore, coating failure was simply unacceptable. To guarantee that this would not happen, the coating manufacturer had to test and approve pre-shipment samples for every candidate lot of resin. These evaluations took three man-days per resin sample. Clearly, this was a very costly problem for both the coating manufacturer and the resin supplier. This coating manufacturer did not understand how normal resin variations could impact performance of their coating. They ignored these subtle details, and it became a costly error for all involved.
Recommendations
• Do not overlook these subtle details. They are not just the purview of the epoxy resin chemist. Good formulators need to understand them, as well. Awareness and understanding of these issues can assist in formulating robust systems and can help to avoid performance failures.
• Do not assume that all epoxy resins are the same. They come from different suppliers using different processes and equipment. Subtle differences in epoxy resins are naturally a result.
• Talk to your epoxy suppliers about these potential differences.
• Get the typical range for the resin characteristics that are not governed by specifications. Suppliers will not usually bring up these issues. The formulator must raise them. This information is useful in comparing resins from different suppliers and can be useful in troubleshooting problems later.
• Get certificates of analysis on all resin lots with which you do development work. This information is also useful in comparing resins from different suppliers and can be useful in troubleshooting problems later.
• Collect certificates of analysis on all resin lots used to make commercial products. Keep these on file. This information can be invaluable in troubleshooting problems later.
• Do not do all development work with a single lot of resin. Use different resin lots, potentially from different suppliers. Use the certificate of analysis data and typical ranges to verify that you are evaluating resins with different characteristics – even though those characteristics are within specification. Ask your suppliers to provide samples with different characteristics. Verify that your formulations are not sensitive to changes in these factors.
• Qualify alternate epoxy suppliers for your formulations. Verify that supply changes will not affect performance. Clearly, formulators are busy and do not always have time to do this. But economic factors can cause supply changes with very little notice. This can be a useful and valuable investment that can help avoid unanticipated and costly coating failures.
For more information, e-mail mwatkins@dixiechemical.com.
This paper was presented at a meeting of the Thermoset Resin Formulators Association at the Crowne Plaza Niagara-Fallsview, Niagara Falls, Ontario, September 12-13, 2011.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!















