New Antimicrobial Meets Performance and Low-VOC Requirements




Fungal spoilage and algal defacement of industrial and private structures has been an ongoing challenge to the coatings industry for decades.1 In recent years, dry film preservation, such as in-can preservation, has evolved such that today it requires formulation expertise paired with regulatory excellence and strict quality control.
Due to increased awareness of the environmental impact of microbicides, stringent requirements have been set, which has resulted in fewer available options for dry film preservation. In addition, important attributes for dry film preservatives, such as low leachability, limit choices further. Additionally, paint manufacturers need support in their efforts to produce low- or zero-VOC coatings.2
Dow Microbial Control spearheaded new technology for dry film preservation by developing the active ingredient 4,5-dichloro-2-n-octyl-4-isothiazoline-3-one (DCOIT), which received the Presidential “Designing Greener Chemicals Award” in 1996 from the U.S. Environmental Protection Agency (EPA).3
More recently, DCOIT was recognized by the European authorities as the first active substance used in Product Type 21 (antifouling products) for which a positive recommendation for Annex I inclusion has been issued.4 This is further proof of the excellent environmental profile of the active ingredient even in view of the most stringent regulations to date. The active ingredient and its metabolites are non-persistent in the environment, and the low solubility in water (3.5 mg/L)5 renders it an ideal film preservative, effective over years in low concentrations.
The EPA-registered DCOIT is also a well-proven mildewcide, providing algaecidal properties. In certain cases, it has been demonstrated that it can contribute to bactericidal efficacy of in-can preservatives, especially against Pseudomonas sp.Its antifungal efficacy is excellent, particularly against Aureobasidium pullulans, which is recognized as the primary fungus responsible for mildew development.6
Longstanding successful products that benefit from the inherent properties of DCOIT are the 20% active formulations, ROZONE™ 2000 Antimicrobial and ROCIMA™ 200 Antimicrobial.
BIOBAN™200, Dow Microbial Control’s new product incorporating DCOIT, takes dispersion development one step further, supporting manufacturers’ and consumers’ growing demand for lower VOC content. BIOBAN 200 also incorporates the proprietary stabilization technique used in ROCIMA 200. Due to technology enhancements, the product has been formulated with negligible VOCs compared to ROCIMA 200, yet keeps its excellent performance characteristics in both exterior and interior coatings applications. It is formaldehyde-release free, does not contain heavy metal-based active ingredients and does not require ZnO for mildewcidal stability. BIOBAN 200 is an aqueous dispersion that is easily incorporated into coatings formulations and it is U.S. EPA registered for use in coatings.
Experiment
Determination of VOCs in Two Water-Based Paints Formulated with BIOBAN 200
• VOC by U.S. EPA method 24.7 This involves heating the coating sample for 1 h at 110 °C and measuring weight loss not accounted for by the water content.
• Water concentration was measured by Karl Fischer titration.
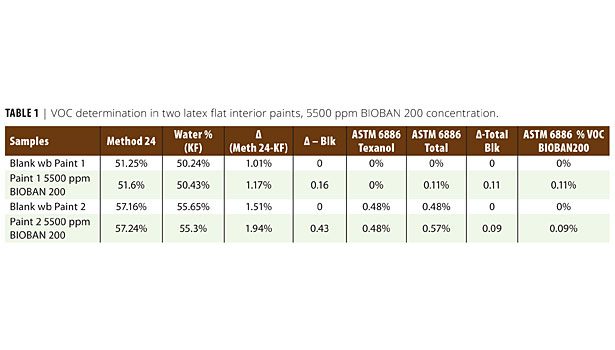
• VOC by ASTM D 6886 test method8 involves dispersion/extraction of a known weight of coating in tetrahydrofuran (THF) or acetone, use of an internal standard, and analysis by gas chromatography (GC) to quantify VOCs and exempt organic compounds, if any, present in the coating. Summation of the individual volatile organic compound weight fractions gives the total VOC content of the coating measured in weight percent. Table 1 shows the low contribution of BIOBAN 200 to the VOC content of two latex flat interior architectural paints.
Dry Film Efficacy of BIOBAN 200 Against Fungi by ASTM Method 55909
Experimental Preparation
• Fungal spores were harvested from mature microorganisms grown on potato dextrose agar.
• Organisms used in this study include: Aspergillus nigerATCC# 6275, Penicillium funiculosumATCC# 11797 and Aureobasidium pullulansATCC# 9348.
• Each spore sample was adjusted to a concentration of ~2 x 106 spores/mL. Equal volumes of each spore suspension were pooled prior to sample inoculation (A. nigerATCC# 6275 and P. funiculosumATCC# 11797 were tested as a pooled inoculum, whereas A. pullulans ATCC# 9348 was tested separately).
Test Method
• The samples were prepared by casting 3-mil wet films of the paint samples on Leneta™ drawdown paper and air dried for 24 h.
• Two 1-inch strips were cut from each dry film. One set of strips was leached for 24 h with distilled water in 1-gal containers at a flow rate of six changes per day and dried again, while the other set remained unleached.
• The coated strips samples were placed on potato dextrose agar.
• 0.5 mL of the fungal inoculum was deposited directly onto the coated samples and evenly spread over the entire substrate/agar surface.
• Agar plates were incubated at 30 °C, with high humidity, for the course of the experiment.
Fungal Growth Measurement
• Plates were scored at seven-day intervals over a 28-day period.
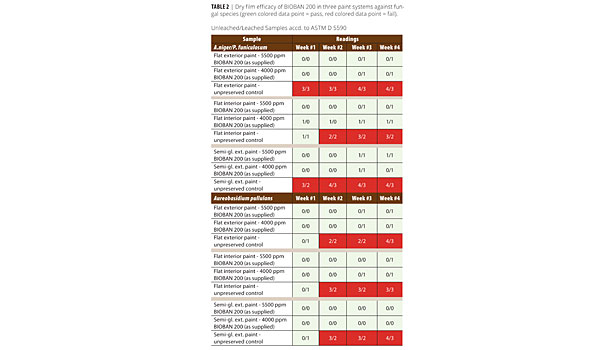
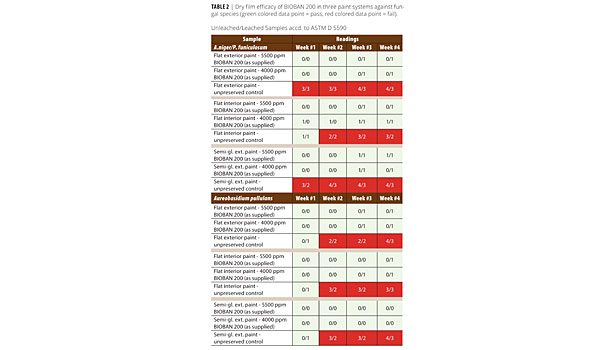
• The readings at week four were used to determine sample pass/fail (see Table 2). Samples with scores of 0 or 1 were considered to have passed the test and are shown in green. Samples with scores 2 and above are considered a fail and are shown in red.
• Scoring scale: No growth = 0; T Trace (<10% coverage) = 1; Light (10 - 30% coverage) =2; Moderate (30 - 60% coverage) = 3; and Heavy (60 - 100% coverage) = 4.
The unleached as well as leached samples show the efficacy of BIOBAN 200 at 800 and 1100 ppm DCOIT active ingredient concentration against a mixture of A. niger/P. funiculosumand A. pullulansin two architectural paints. The unleached samples exhibit a maximum <10 % rating, most specimens are clean of fungal growth. The untreated paints readily support fungal growth.
The leached samples show the potency and low leachability of the active ingredient DCOIT. There are no significant differences between the leached and unleached series.
Outdoor Exposure Results
A 100% acrylic latex semigloss paint was prepared. The formulation contained a TiO2 slurry as the sole pigment and used HEUR rheology modifiers to control viscosity. The final formulation had a pigment volume concentration of 22% with 34% volume solids. Various biocides were post-added to the finished paint with adequate agitation provide by an overhead laboratory mixer. Panel preparation was completed under controlled conditions. The primer used in these experiments was a biocide-free acrylic stain-blocking primer. One coat was applied to the white pine substrate followed by two coats of test paint. The cedar substrate received two coats of primer. All paints were brush applied using a spread rate of 400 sq ft/gal, with 24 h between coats. All panels were exposed in Glen St. Mary, FL, in a north vertical (NV) orientation.
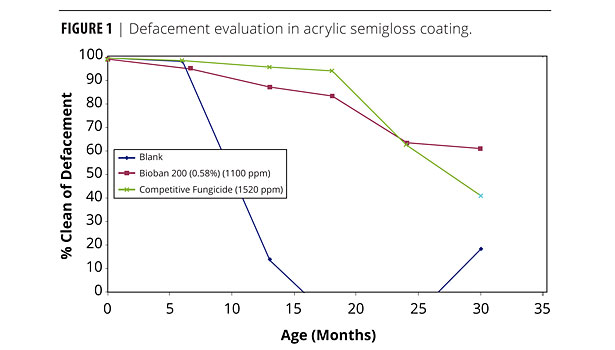
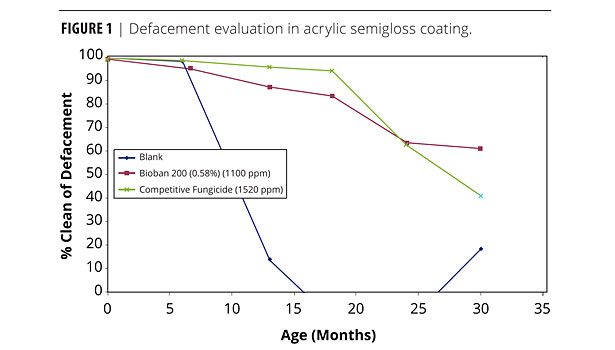
Figure 1 shows the graphical evaluation of the overall defacement over time in an acrylic semigloss coating exposed in Glen St. Mary, FL, on primed white pine. After seven months, the blank sample deteriorated quickly to total defacement by 16 months.
BIOBAN 200 showed just 10% defacement after 14 months and stabilized at 30% defacement after 30 months, whereas a competitive microbicidal active deteriorated quickly after 18 months (initially 5% defacement) to 60% defacement.
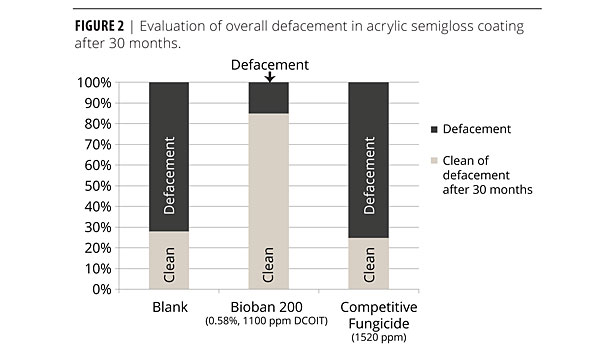
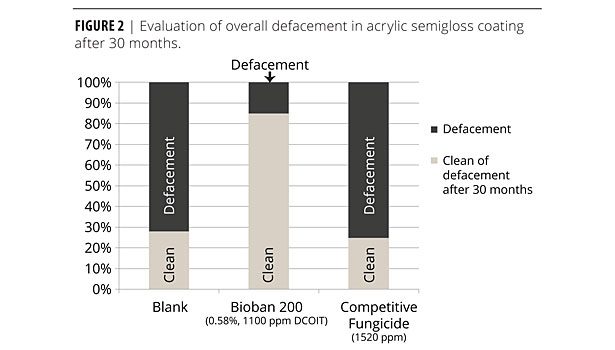
Figure 2 exhibits a similar picture. Here, the substrate was changed to primed cedar, exposure conditions identical to Figure 1. After 30 months, BIOBAN 200 at 1100 ppm outperformed the competitive fungicide in higher concentration by far. Eighty five percent of the panels were free of defacement, whereas the competitive mildewcide deteriorated to a value similar to the untreated panel (25- 30% free of defacement).
Again, the DCOIT-based BIOBAN 200 showed remarkable performance because of its inherent low leaching characteristics and excellent microbicidal efficacy.
Summary
The proprietary stabilization of the active ingredient DCOIT paired with proven microbicidal efficacy and low leaching characteristics provides the remarkable performance characteristics of BIOBAN 200. Further, BIOBAN 200 supports the growing demand of architectural paint manufacturers and consumers for lower-VOC-content products. n
References
1 Warscheid, T. Impacts of microbial biofilms in the deterioration of inorganic building materials and their relevance for the conservation practice, Internationale Zeitschrift für Bauinstandsetzen 2 (6), (1996) 493-504.
2 Köhler, T.; Carlsen A.; Tiedtke, G. Breakthrough innovation for in-can preservation, PPCJ(201), 4563, 28-29, (2011).
3 United States Environmental Protection Agency, The Presidential Green Chemistry Challenge Awards Program, Summary of 1996 Award Entries and Recipients, http://www.epa.gov/greenchemistry/pubs/docs/award_entries_and_recipients1996.pdf, p.4 (EPA744-K-96-001).
4 Dow Microbial Control Press Release: DCOIT, an Active Substance of Dow Microbial Control, recommended by the EU Competent Authority for approval and inclusion in BPD Annex I listing under PT 21: Antifouling Products. http://www.dow.com/microbial/news/2011/20110329a.htm
5 Dow Microbial Control, Material Safety Data Sheet (2011), KATHON™ 287T biocide, sect. 9.
6 Sadasivan, L; Gandhi, U. A “two-for-one” biocide for latex coatings: provides effective in-can and dry film preservation. PCI Magazine, July, (2003).
7 EPA Test Method 24: Determination of volatile matter content, water content, density, volume solids, and weight solids of surface coatings, p.1180-1194.
http://www.epa.gov/ttn/emc/promgate/m-24.pdf
8 ASTM D 6886 (2003) Standard Test Method for Speciation of the Volatile Organic Compounds (VOCs) in Low VOC Content Waterborne Air-Dry Coatings by Gas Chromatography.
9 ASTM D 5590-00 (2005) Standard Test Method for Determining the Resistance of Paint Films and Related Coatings to Fungal Defacement by Accelerated Four-Week Agar Plate Assay.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!