The Cost-Saving Benefits of Environmental Durability and Performance Testing
The subject of product environmental durability usually comes up as an afterthought, often only following a major problem or customer complaint. However, it is far less costly in terms of time, money and customer relations to understand and avoid risk up front than it is to deal with the failed consequences.

A major manufacturer of contract office furnishings was suddenly faced with a series of product discoloration issues. In particular, one color of powder coated steel office furniture resulted in more than $400,000 in warranty costs during one year alone. Worse, the problem wasn’t predicted by the manufacturer’s in-house testing program, and the scope of the problem and potential liability over the lengthy warranty period (over 10 years) was undetermined.
Look around and you’ll see numerous finishing performance issues. Discolorations of famous-name anodized MP3 players, yellowing of appliance control panels, faded coatings on bathroom fixtures…there is no shortage of examples.
The subject of product environmental durability usually comes up as an afterthought, often only following a major problem or customer complaint. This is unfortunate as it comes at a time when the problem is too late to be fixed, the entire scope of which may not be completely understood, and the result goes right to (or rather out of) the bottom line. Perhaps even worse than not knowing if you have a potential issue (the “ignorance is bliss” approach) is having a testing program in place that doesn’t predict problems. Quite frankly, many finishers and manufacturers believe they have a predictive quality program in place when in fact they have just been lucky. However, it is far less costly in terms of time, money and customer relations to understand and avoid risk up front than it is to deal with the failed consequences.
Change is constantly with us. Many finish failures are the result of changes to the formulation. Increased volatile organic compound (VOC) regulations have changed the nature of coatings, often from solvent-based to waterborne high-solids formulations, powder coatings, ultraviolet (UV) and radiation-cure systems, and others with different performance and durability levels. Colorant changes, such as the elimination of heavy-metal and hexavalent chromium pigments in favor of less lightfast, high-chroma organic ones, have been a major issue. The office equipment discoloration mentioned earlier was directly related to a change in pigment chemistry as part of a corporate “go green” management initiative that had unexpected consequences.
The service environment of many products is also changing. Customers have a habit of using products in applications and geographies for which they weren’t originally envisioned, designed or tested. Many household appliances, for example, are no longer boxy “white goods” but are bold and colorful décor statements showcased in visible, well-lit locations such as the kitchen. The nature of the interior environment features increased natural daylight with window walls and skylights, even in “big box” retail locations, homes and offices. And the “mobile generation” of finished products now sees greater outdoor and automotive interior exposure than before.

We must first understand why we test. There are two fundamental and often opposite reasons for testing. The first is because we have to, most commonly because we have a customer specification, industry requirement, or other compelling reason if we want to sell our product or service. Testing is always a burden and expense, right? So we want to get by (meaning pass the test) as easily and inexpensively as we can. Testing is a hurdle, a price of admission that we must bear to get on with business.
These tests or specifications may or may not be meaningful. There are a lot of customer and industry specs that really are not an indicator of meaningful product performance or durability.
The other reason for testing is to understand a product or process. What are the limits of our processing window? Can we change ingredient suppliers or the formulation without affecting (or maybe actually improving) product performance? Is our product better or worse than our previous one or our competitors’ product? Can we factor out costs, speed up the line, increase our warranty, broaden our market, find new applications and understand the consequences? Can we mitigate risk and not bet on blind luck? Testing can help us truly understand our business and our product.
So what kind of “environmental durability and performance” tests should you consider? Again, you may need to perform some tests because of a requirement that can’t be avoided. The trick is to understand the limits and biases of those tests so that you understand what the results are really telling you. You might be able to get a lot more information from a reasonable test, with little or no additional cost, with a well-planned program. Such a program may mean more or different sample types, such as a set with different levels of additives to see where you can remove costs. Or it may require additions to the test, such as one more temperature on a heat stability test to indicate how your product may perform in a different environment, or during transport and storage - data that sales and marketing might use.
Environmental exposure tests usually evaluate primarily color and appearance (C&A) properties or performance. For example, C&A may be yellowing of a coating, loss of gloss on a finish, color fade or hue shift, surface cracking, delamination, or other defects. These problems may be the result of the effects of artificial lighting or sunlight, outdoor weathering, heat and moisture exposure, exposure to cleaners and chemicals such as chlorinated water, insect repellants and suntan lotion, oil, atmospheric pollutants and ozone, or they may result from additives migrating to the surface under temperature or pressure, etc. For manufactured products, such changes might also result from dissimilar materials in contact with one another. Changes in C&A may also be caused by scrubbing, washing or other maintenance.
Performance characteristics are physical and are generally more closely related to the “bulk properties” of a material than to the result of surface changes, as is typical with C&A. However, physical performance can be affected. A coating exposed to natural or artificial light or heat can experience increased crosslinking, which results in embrittlement with exposure. Such embrittlement can lead to coating fractures and delamination (disbonding) upon impact, or to corrosion of a metal substrate.
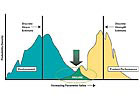
One fundamental rule of testing is to expose a product to the maximum worst-case environmental stresses it is expected to see in order to predict true performance (see Figure 1). This is an extremely important concept and has two major consequences if it is not followed. One negative consequence of environmental exposure that we can detect (C&A and mechanical property changes) is the physical manifestation of fundamentally “bad” chemistry. Chemical reactions have certain energies (called activation energies) associated with them. Think of them as “trigger” points. If you don’t reach the trigger level, such as testing at too low a temperature compared to the real environment, you don’t cause the “bad” chemistry to occur. The product may pass the test but fail in real life.
The other extreme can be detrimental as well. One common fallacy in testing is that if you really “beat on” a product with an extremely harsh test, and it passes, then it will survive anywhere. The worst consequence is that the test may fail potentially good products, but that has two outcomes. You may have to explore the results more closely to see if the product is really okay, and more importantly, you won’t risk putting out product that may fail and result in real out-of-pocket money.
This sounds like a good strategy, but it is flawed. The reality is that more severe tests are different tests than the real world, and doing a different test may result in different results. The clearcoat paint that fell off of cars in the 1980s was the result of this kind of approach. We now understand that a more severe stress can change the chemistry of what happens, and the results can be both unpredicted and surprising. So it is important to stress the product at maximum, but realistic, levels.
Another aspect of environmental exposure testing has to do with design strength and test stress. All products are designed with a set of desired or required properties. For a coating, these properties may be a required applied thickness, crosslink density, flow and leveling to fill substrate surface defects, gloss and initial color match, etc. The design strength will be greater than the expected stresses, such as the surface roughness of the substrate or the amount of solar radiation that will make the pigment fade, with some excess in strength to allow for a safety margin. However, no product as actually manufactured will have a perfect match to the design strength. Due to raw ingredient and process variability, for example, the strength as delivered is actually a distribution. Great products have a tight distribution with little variability, others less so.
So, too, the service environment any product sees is a distribution. One washing machine may only see a basement laundry room in Minnesota, while another will experience the exposed porch of a seaside home in the Southeast. Where the lower strength distribution of product as manufactured meets the higher stress of the range of environments, failure of even well designed products can occur. Proper testing can evaluate this possibility.
One additional aspect of environmental durability and performance testing relates to “infant mortality.” In reliability theory, this principle refers to the products that fail “out of the box” or very early in their life, far earlier than the normal wear-out aging of the bulk of the product. These special cause failures are usually due to abnormal product formulation or processing, such as a key ingredient was left out, or the process was well out of tolerance, resulting in some product prematurely failing. This scenario is particularly bad, since it results in both the majority of warranty claims and loss of brand quality image. “Infant mortality” testing requires a somewhat different testing plan than initial specification conformance or durability performance assessment as it involves ongoing surveillance testing of product throughout its manufacturing life. However, once the product is characterized, accelerated screening tests can be used to minimize costs and time.

Generally, the worst-case environment for these kinds of products is a hot dry or hot wet outdoor environment. For example, most polymeric materials and coatings are outdoor tested by exposing them to the environment of subtropical South Florida. Many major paint companies have their own exposure test sites located there, as do commercial exposure labs. The hot, very wet climate with high solar radiation is one of the most damaging, but still realistic, environments. For products that are also sensitive to high temperatures and solar radiation, desert sites in the Southwest U.S. are ideal exposure environments. For products destined for other environments, a variety of specialized exposure sites, including Northern industrial, cold weather, coastal, and high altitude are also available.
Outdoor exposure tests can also be conducted behind glass, such as automotive or architectural glass, for indoor exposure conditions, and special tests can be designed for unique applications. Since the conditions of outdoor testing can be monitored but not really controlled, repeatable and reproducible laboratory environmental exposures - also known as “artificial” or “accelerated” weathering - can be conducted with exposure chambers. Tests under controlled conditions of lighting (such as outdoor direct sunlight or daylight filtered through window glass), temperature, humidity and rain can be done in the laboratory to true outdoor or indoor conditions. These “accelerated” tests can be particularly useful in that a variety of environments can be created in a fast timeframe.
One aspect of these types of tests is that they can either be done as needed in commercial labs or brought in-house for ongoing work. Another aspect is that instead of reproducing any single outdoor environment, they can be adjusted to bracket the “worst case” expected combinations of light, temperature and moisture conditions in a single test exposure, thus reducing test time and expense while at the same time minimizing the risk of product failure. A variety of test instruments are available with various environmental simulation capabilities, sizes and technologies.
For materials that are known to be temperature- or temperature- and moisture-sensitive, aging ovens and temperature/humidity climate chambers without solar light exposure are effective. Surface degradation resulting in yellowing, for example, is often heat related. Climate chambers typically can be operated at setpoints, or temperatures can be stepped or ramped at controlled rates to provide thermal cycling, which can affect materials through by thermal expansion and contraction. One major manufacturer of furnishings was able to catalog standard combinations of colorants, coatings and plastics to understand their performance so that each new product did not have to be tested individually in each color.
For metal substrates and metalized products, salt air corrosion can be an issue. While traditional steady-state tests to continuous salt fog/salt spray exposure have been traditional for decades, they often don’t correlate to real-world performance. We have come to realize that much of the “bad” chemistry that happens occurs during “transition” periods, e.g., from wet to dry, hot to cold, etc. Newer versions of salt corrosion fog chambers incorporating wet/dry cycles have greatly improved the correlation of lab tests to real-world performance.
Exposure to common chemicals such as cleaning agents and personal care products can usually be conducted without special equipment. Some common household cleaners and chemicals such as ammonia and bleach can attack finishes, as can the insect repellant DEET and PABA found in sunscreen lotions. By screening your product’s reaction to some common chemicals, you can understand the product’s sensitivities and decide how to improve the finish or limit your liability.
Atlas Material Testing Technology LLC’s website is at www.atlasmts.com.

Artificial exposure tests can be adjusted to bracket the “worst case” expected combinations of light, temperature and moisture conditions in a single test exposure.
A major manufacturer of contract office furnishings was suddenly faced with a series of product discoloration issues. In particular, one color of powder coated steel office furniture resulted in more than $400,000 in warranty costs during one year alone. Worse, the problem wasn’t predicted by the manufacturer’s in-house testing program, and the scope of the problem and potential liability over the lengthy warranty period (over 10 years) was undetermined.
Look around and you’ll see numerous finishing performance issues. Discolorations of famous-name anodized MP3 players, yellowing of appliance control panels, faded coatings on bathroom fixtures…there is no shortage of examples.
The subject of product environmental durability usually comes up as an afterthought, often only following a major problem or customer complaint. This is unfortunate as it comes at a time when the problem is too late to be fixed, the entire scope of which may not be completely understood, and the result goes right to (or rather out of) the bottom line. Perhaps even worse than not knowing if you have a potential issue (the “ignorance is bliss” approach) is having a testing program in place that doesn’t predict problems. Quite frankly, many finishers and manufacturers believe they have a predictive quality program in place when in fact they have just been lucky. However, it is far less costly in terms of time, money and customer relations to understand and avoid risk up front than it is to deal with the failed consequences.
Change is a Constant
What is the “environmental durability” of a finished part or product? Quite simply, it is the negative effect of the environment on product appearance, performance or both during some anticipated lifetime, such as the warranty period or other reasonable customer expectation. The product lifetime not only includes the end use service environment, wherever that may be, but it also can include the interim manufacturing, transportation and storage, and point of sale environment.Change is constantly with us. Many finish failures are the result of changes to the formulation. Increased volatile organic compound (VOC) regulations have changed the nature of coatings, often from solvent-based to waterborne high-solids formulations, powder coatings, ultraviolet (UV) and radiation-cure systems, and others with different performance and durability levels. Colorant changes, such as the elimination of heavy-metal and hexavalent chromium pigments in favor of less lightfast, high-chroma organic ones, have been a major issue. The office equipment discoloration mentioned earlier was directly related to a change in pigment chemistry as part of a corporate “go green” management initiative that had unexpected consequences.
The service environment of many products is also changing. Customers have a habit of using products in applications and geographies for which they weren’t originally envisioned, designed or tested. Many household appliances, for example, are no longer boxy “white goods” but are bold and colorful décor statements showcased in visible, well-lit locations such as the kitchen. The nature of the interior environment features increased natural daylight with window walls and skylights, even in “big box” retail locations, homes and offices. And the “mobile generation” of finished products now sees greater outdoor and automotive interior exposure than before.

A faded coating on door handles.
Why Test?
So what’s a manufacturer or finisher to do? Surely you can’t be expected to test everything for every conceivable exposure location and environmental condition. And aren’t there standards such as ASTM test methods to rely on for protection? The answers to these questions depend on a lot of business and technical considerations.We must first understand why we test. There are two fundamental and often opposite reasons for testing. The first is because we have to, most commonly because we have a customer specification, industry requirement, or other compelling reason if we want to sell our product or service. Testing is always a burden and expense, right? So we want to get by (meaning pass the test) as easily and inexpensively as we can. Testing is a hurdle, a price of admission that we must bear to get on with business.
These tests or specifications may or may not be meaningful. There are a lot of customer and industry specs that really are not an indicator of meaningful product performance or durability.
The other reason for testing is to understand a product or process. What are the limits of our processing window? Can we change ingredient suppliers or the formulation without affecting (or maybe actually improving) product performance? Is our product better or worse than our previous one or our competitors’ product? Can we factor out costs, speed up the line, increase our warranty, broaden our market, find new applications and understand the consequences? Can we mitigate risk and not bet on blind luck? Testing can help us truly understand our business and our product.

Figure 1. One fundamental rule of testing is to expose a product to the maximum worst-case environmental stresses it is expected to see in order to predict true performance.
Develop a Testing Program Plan
Okay, so maybe you’re willing to consider reevaluating your testing program. But isn’t increased testing more costly? The short answer may rightly be “Yes, it can be, but what is the value of the testing you’re now doing if you can’t rely on it to make meaningful business decisions?” Probably true, but you could never convince your boss, right? The goal is to maximize the amount of truly useful information from a minimal amount of testing. Achieving this goal requires a testing program plan.So what kind of “environmental durability and performance” tests should you consider? Again, you may need to perform some tests because of a requirement that can’t be avoided. The trick is to understand the limits and biases of those tests so that you understand what the results are really telling you. You might be able to get a lot more information from a reasonable test, with little or no additional cost, with a well-planned program. Such a program may mean more or different sample types, such as a set with different levels of additives to see where you can remove costs. Or it may require additions to the test, such as one more temperature on a heat stability test to indicate how your product may perform in a different environment, or during transport and storage - data that sales and marketing might use.
Environmental exposure tests usually evaluate primarily color and appearance (C&A) properties or performance. For example, C&A may be yellowing of a coating, loss of gloss on a finish, color fade or hue shift, surface cracking, delamination, or other defects. These problems may be the result of the effects of artificial lighting or sunlight, outdoor weathering, heat and moisture exposure, exposure to cleaners and chemicals such as chlorinated water, insect repellants and suntan lotion, oil, atmospheric pollutants and ozone, or they may result from additives migrating to the surface under temperature or pressure, etc. For manufactured products, such changes might also result from dissimilar materials in contact with one another. Changes in C&A may also be caused by scrubbing, washing or other maintenance.
Performance characteristics are physical and are generally more closely related to the “bulk properties” of a material than to the result of surface changes, as is typical with C&A. However, physical performance can be affected. A coating exposed to natural or artificial light or heat can experience increased crosslinking, which results in embrittlement with exposure. Such embrittlement can lead to coating fractures and delamination (disbonding) upon impact, or to corrosion of a metal substrate.
One fundamental rule of testing is to expose a product to the maximum worst-case environmental stresses it is expected to see in order to predict true performance (see Figure 1). This is an extremely important concept and has two major consequences if it is not followed. One negative consequence of environmental exposure that we can detect (C&A and mechanical property changes) is the physical manifestation of fundamentally “bad” chemistry. Chemical reactions have certain energies (called activation energies) associated with them. Think of them as “trigger” points. If you don’t reach the trigger level, such as testing at too low a temperature compared to the real environment, you don’t cause the “bad” chemistry to occur. The product may pass the test but fail in real life.
The other extreme can be detrimental as well. One common fallacy in testing is that if you really “beat on” a product with an extremely harsh test, and it passes, then it will survive anywhere. The worst consequence is that the test may fail potentially good products, but that has two outcomes. You may have to explore the results more closely to see if the product is really okay, and more importantly, you won’t risk putting out product that may fail and result in real out-of-pocket money.
This sounds like a good strategy, but it is flawed. The reality is that more severe tests are different tests than the real world, and doing a different test may result in different results. The clearcoat paint that fell off of cars in the 1980s was the result of this kind of approach. We now understand that a more severe stress can change the chemistry of what happens, and the results can be both unpredicted and surprising. So it is important to stress the product at maximum, but realistic, levels.
Another aspect of environmental exposure testing has to do with design strength and test stress. All products are designed with a set of desired or required properties. For a coating, these properties may be a required applied thickness, crosslink density, flow and leveling to fill substrate surface defects, gloss and initial color match, etc. The design strength will be greater than the expected stresses, such as the surface roughness of the substrate or the amount of solar radiation that will make the pigment fade, with some excess in strength to allow for a safety margin. However, no product as actually manufactured will have a perfect match to the design strength. Due to raw ingredient and process variability, for example, the strength as delivered is actually a distribution. Great products have a tight distribution with little variability, others less so.
So, too, the service environment any product sees is a distribution. One washing machine may only see a basement laundry room in Minnesota, while another will experience the exposed porch of a seaside home in the Southeast. Where the lower strength distribution of product as manufactured meets the higher stress of the range of environments, failure of even well designed products can occur. Proper testing can evaluate this possibility.
One additional aspect of environmental durability and performance testing relates to “infant mortality.” In reliability theory, this principle refers to the products that fail “out of the box” or very early in their life, far earlier than the normal wear-out aging of the bulk of the product. These special cause failures are usually due to abnormal product formulation or processing, such as a key ingredient was left out, or the process was well out of tolerance, resulting in some product prematurely failing. This scenario is particularly bad, since it results in both the majority of warranty claims and loss of brand quality image. “Infant mortality” testing requires a somewhat different testing plan than initial specification conformance or durability performance assessment as it involves ongoing surveillance testing of product throughout its manufacturing life. However, once the product is characterized, accelerated screening tests can be used to minimize costs and time.

Repeatable and reproducible laboratory environmental exposures, also known as “artificial” or “accelerated weathering,” can be conducted with exposure chambers.
Determine the Testing Methods
One of the main mechanisms for the deterioration of organic materials, such as decorative and protective paints and coatings and powder coatings, is degradation resulting from the effects of heat, light and moisture, either individually or in combination. This degradation often results in discoloration, especially yellowing, embrittlement and surface gloss loss. In extreme cases, delamination, cracking or hazing can also result. In the case of colored coatings or dye anodized or metalized finishes, the colorant may also be destroyed, leading to color fade or hue shift.Generally, the worst-case environment for these kinds of products is a hot dry or hot wet outdoor environment. For example, most polymeric materials and coatings are outdoor tested by exposing them to the environment of subtropical South Florida. Many major paint companies have their own exposure test sites located there, as do commercial exposure labs. The hot, very wet climate with high solar radiation is one of the most damaging, but still realistic, environments. For products that are also sensitive to high temperatures and solar radiation, desert sites in the Southwest U.S. are ideal exposure environments. For products destined for other environments, a variety of specialized exposure sites, including Northern industrial, cold weather, coastal, and high altitude are also available.
Outdoor exposure tests can also be conducted behind glass, such as automotive or architectural glass, for indoor exposure conditions, and special tests can be designed for unique applications. Since the conditions of outdoor testing can be monitored but not really controlled, repeatable and reproducible laboratory environmental exposures - also known as “artificial” or “accelerated” weathering - can be conducted with exposure chambers. Tests under controlled conditions of lighting (such as outdoor direct sunlight or daylight filtered through window glass), temperature, humidity and rain can be done in the laboratory to true outdoor or indoor conditions. These “accelerated” tests can be particularly useful in that a variety of environments can be created in a fast timeframe.
One aspect of these types of tests is that they can either be done as needed in commercial labs or brought in-house for ongoing work. Another aspect is that instead of reproducing any single outdoor environment, they can be adjusted to bracket the “worst case” expected combinations of light, temperature and moisture conditions in a single test exposure, thus reducing test time and expense while at the same time minimizing the risk of product failure. A variety of test instruments are available with various environmental simulation capabilities, sizes and technologies.
For materials that are known to be temperature- or temperature- and moisture-sensitive, aging ovens and temperature/humidity climate chambers without solar light exposure are effective. Surface degradation resulting in yellowing, for example, is often heat related. Climate chambers typically can be operated at setpoints, or temperatures can be stepped or ramped at controlled rates to provide thermal cycling, which can affect materials through by thermal expansion and contraction. One major manufacturer of furnishings was able to catalog standard combinations of colorants, coatings and plastics to understand their performance so that each new product did not have to be tested individually in each color.
For metal substrates and metalized products, salt air corrosion can be an issue. While traditional steady-state tests to continuous salt fog/salt spray exposure have been traditional for decades, they often don’t correlate to real-world performance. We have come to realize that much of the “bad” chemistry that happens occurs during “transition” periods, e.g., from wet to dry, hot to cold, etc. Newer versions of salt corrosion fog chambers incorporating wet/dry cycles have greatly improved the correlation of lab tests to real-world performance.
Exposure to common chemicals such as cleaning agents and personal care products can usually be conducted without special equipment. Some common household cleaners and chemicals such as ammonia and bleach can attack finishes, as can the insect repellant DEET and PABA found in sunscreen lotions. By screening your product’s reaction to some common chemicals, you can understand the product’s sensitivities and decide how to improve the finish or limit your liability.
Improve Your Bottom Line
Trusting blind luck is not the best way to improve your products and processes, expand your markets and applications, or improve your bottom line. Neither is testing without a plan. Customer specifications and standard test methods may offer a place to start, but no one understands your product and your needs better than you. Numerous sources, including materials specialists, commercial labs and testing specialists, are available to help you make the best use of your testing dollars and send more of them to your bottom line.Atlas Material Testing Technology LLC’s website is at www.atlasmts.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



