Leveling Additives Affect Film Formation of Powder Coatings
Adhesion quality and optical appearance of coatings are determined by film formation. Several factors, including application parameters, rheological behavior and interfacial properties, interact in a complicated manner (1). Simply put, surface tension can be regarded as the driving force and viscosity as the resistance of wetting and leveling (2-5). This article discusses the interfacial properties. The rheological behavior of powder coating melts, as well as the influence of different application parameters (e.g. particle-size distribution, film thickness, etc.) on film formation are not discussed.
Wetting of a substrate and leveling of a fluid film strongly depend on the surface tension of the coating. However, both processes have opposing requirements in terms of surface tension. If the surface tension is too high, poor wetting occurs, which leads to defects such as craters. On the other hand, if the surface tension is too low, the leveling is adversely influenced, which leads to wavy surfaces known as orange peel (6). Moreover, surface flow can cause surface defects due to local surface-tension differences, known as the Marangoni Effect (7,8). These differences can arise from temperature gradients and local inhomgenieties (e.g. contaminations). Therefore, the surface tension of a coating must be controlled and adjusted (9,10).
The surface tension of powder coatings can be adjusted by incorporating leveling additives to a coating formulation. In essence, these additives are surface-active materials that decrease the surface tension of the coating. An optimum range can be adjusted, and local surface tension differences are minimized (9,10). However, most current knowledge regarding the effect and surface activity of leveling additives in powder coatings is based on trial and error. Very little reliable surface tension data are available. A more fundamental and quantitative understanding is necessary to develop new, superior additives for improved powder coating formulations.
Information on this subject is scarce for two reasons. First, surface-tension measurements of polymer melts are not trivial, due to high melt viscosities, high measuring temperatures and limited thermal stability of many polymers. Second, powder coatings are complex multicomponent systems that include binders, curing agents, additives, pigments and fillers. The complexity causes additional difficulties in melt surface-tension measurements. These measurements are even more difficult for reactive systems because of the cross-linking reaction. Therefore, to quantify the effect of leveling additives on melt sur-face tension, sophisticated measuring techniques are needed.
The influence of additive properties such as molecular weight and chemical structures (polyacrylates and polyester-modified polysiloxanes) on surface tension of typical powder coating binders was the subject of a study at the Institute of Polymer Research (Dresden, Germany). The study began with nonreactive powder coating systems (i.e., pure binders) (11,12). A suitable technique for measuring surface tension of polymer melts is Axisymmetric Drop Shape Analysis (ADSA) (13,14). This method was used to systematically study the relationship between chemical structures of selected leveling additives (laboratory products) and their influence on melt surface tension of a powder coating binder (15).
Further insight into the effectiveness of leveling additives was obtained by means of AFM phase imaging and viscosity measurements. AFM phase imaging provides qualitative information about the distribution of the additives at the binder surface (16). Viscosity measurements were taken to determine whether leveling additives would affect the viscosity of the binder. The rather complex rheological behavior of a powder coating melt during film formation is beyond the scope of the present investigation.
This article explains the effect of leveling additives in powder coatings. The
results should provide helpful information for choosing appropriate additives
and for planning the synthesis of new, improved additives.

The Experiment Materials
DER 664 UE (Dow, Germany), an epoxy resin based on bisphenol-A with a molecular weight of Mw = 2000 g/mol, was chosen as a typical powder coating binder for this study. All additives were obtained from Byk-Chemie (Wesel, Germany).For a systematic investigation of the effect of molecular weight and molecular structure of the additives on surface tension of the powder coating binder, three different groups of additives were used:
- three homopolymeric n-butylacrylates with different molecular weights
- two copolymeric acrylates based on 2-ethylhexylacrylate with different co-monomers
- two polyester-modified methylalkylpolysiloxanes. Both siloxanes possess the same polyester modification and the same siloxane backbone, but they have different alkyl side chains.


The coated panels were then melted under an IR lamp until a surface temperature of 140°C was reached and then cured immediately. The panels were cured in an Aetek exposure unit by placing them on a moving belt at different speeds and passing them under 2- by 80-Watt/cm Fusion type H medium-pressure Hg-vapor UV-lamps. The temperature of the panels leaving the conveyor was about 130°C. With the BAPO photoinitiator, it is possible to use normal, undoped mercury lamps.
The physical properties of the steel and MDF panels were tested when they had cooled (30 minutes after curing). The results are as follows:
König pendulum hardness. An average of three determinations according to DIN 53157 was
taken.
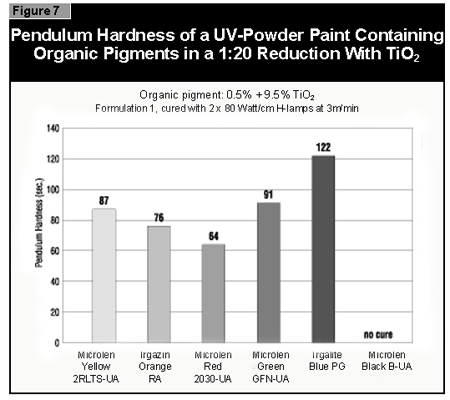
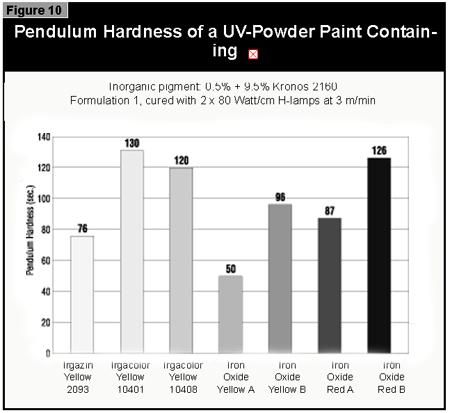
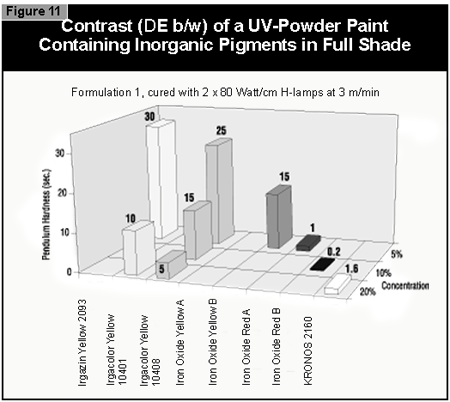
The pendulum hardness values for the coatings made with organic pigments in full shade and as 1:20 redu ctions with rutile are shown in Figures 6 and 7, respectively. With a decrease in pigment concentration, an increase in the hardness is seen as expected. Surprisingly, black is a color that is possible to cure with UV light. A more difficult color to cure is gray, because the TiO2 absorbs the UV-light and the black absorbs the remaining visible light, leaving very little light for the photoinitiator.
If a pendulum hardness of about 100 seconds is taken as the minimum acceptable
cure, this value is reached at 4% pigment concentration by Microlen Yellow
2RLTS-UA, Irgazin DPP Orange RA and Irgalite Blue PG. At 2%, this value is
reached by Microlen Green GFN-UA; at 0.5%, by Microlen Black B-UA.

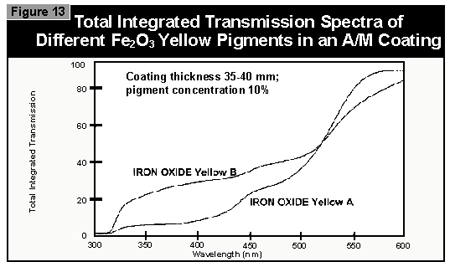
Gallium-doped UV lamps have a spectral output that is greater in the visible region of the spectrum than undoped mercury-vapor UV lamps. It was therefore expected that a better through-cure would be obtained for the yellow pigments, which absorb at the same wavelength as the BAPO photoinitiator (3). When pigmented coatings were cured using either two gallium-doped lamps on their own or the combination of a gallium-doped lamp and an undoped lamp, only minimal improvement of the pendulum hardness was found. This was well below the limits of error (±15 sec.) of the pendulum hardness measurements. It is possible that differences in lamp output and the geometry and construction of the exposure equipment are of importance. Other workers report better cure results with the gallium-doped lamps in white (3) and gray (4) pigmented systems, but this was not found with the equipment used in this study.
The question arises as to why some pigments allow better UV-curing than others, even though the colors of the pigments are nearly identical. For optimal cure, a maximum amount of light in the region of the spectrum at which the photoinitiators absorb must reach the base of the coating. This is a complex problem because the extent of cure depends on:
- spectral output of the lamp
- absorption spectra of the photoinitiators
- quantum yield for radical formation of the photoinitiators--this can be expected to be wavelength dependent
- absorption of light of the wavelength range of interest by all species other than the photoinitiator
- extent of light scattering by the pigment. This increases the path length of the light through the coating and hence the probability of absorption.
- reflectivity of the coating substrate.
Summary and Recommendations
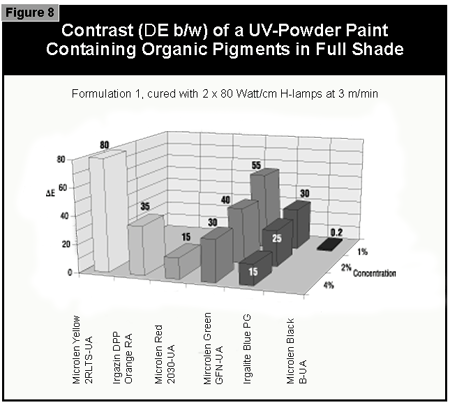
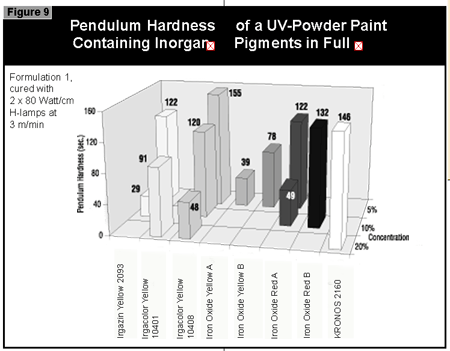
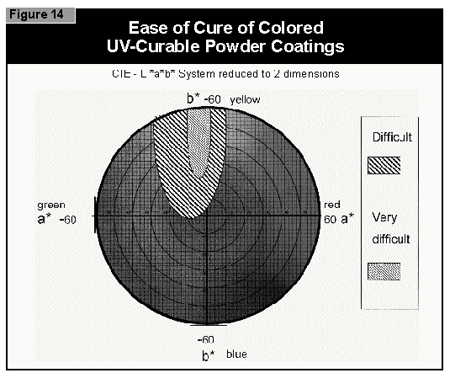
In general, the most difficult color to cure is yellow. At present, it is not possible to cure this color if a high color strength is required. In reductions with TiO2, it is possible to cure yellows at low color strength. The neighboring colors in the color space diagram (orange, brown, some greens and gray) are also difficult to cure (Figure 14).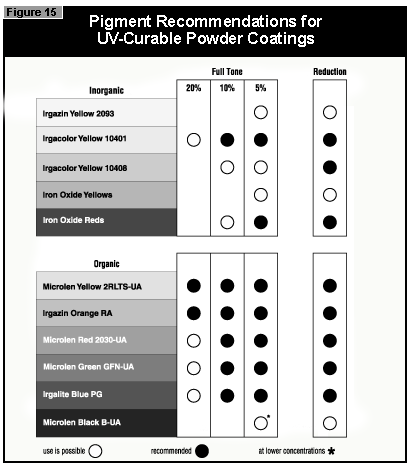
In curing colored pigmented powder coatings, it is essential that enough light can be absorbed by the photoinitiator at the bottom of the coating. A higher ratio of BAPO:AHK of 4:1 is therefore recommended, rather than the 1:1 ratio commonly used for white pigmented systems, but at the same total concentration of 2.5%.
This paper was originally presented at Powder Coating Europe 2000, sponsored by Vincentz Verlag.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



