Measuring the Reaction Kinetics of Polyester/Primid XL-552 Powder Coatings With Dielectric Analysis (DEA)
Dielectric analysis (DEA)
has been used since the mid 1930s for the analysis of various polymeric
materials. In the last few years, DEA has been used increasingly for epoxy resin
systems.
Material and Apparatus
- Primid XL-552 (Bis-N,N-Dihydroxyethyladipamide), EMS-Primid
- Carboxyl polyester with acid value 30, UCB Chemicals
- Polyester/TGIC and polyester/Primid powder coatings, EMS-Primid
- Dielectrometer TA 2970, TA Instruments GmbH
- Differential Scanning Calorimeter, Polymer Laboratories
Method
The powder coating sample was applied to the sensor with a spatula at room temperature. The coated sensor was then placed in the DEA kiln and raised quickly to the required temperature (within approximately 5 minutes). The resulting cross-linking reaction was investigated at dif-ferent temperatures using a ceramic single-surface sensor isotherm. The measurements were made under a nitrogen atmosphere.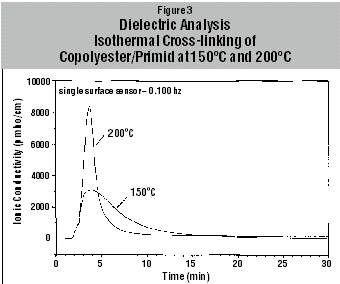
Dielectric Analysis
With Dielectric Analysis the real component e' and the imaginary component e" of the complex dielectric constant e*= e' - ie" are determined in relationship to temperature and the frequency of the alternating electric field. The real component e' is proportional to the reversible stored energy, and the imaginary component (or loss factor) e" is proportional to the dissipated energy resulting from the rotation of polar groups.The Primid cross-linking reaction was traced by following the ionic conductivity s:s = e" 2P f eo where f is the frequency in Hz of the alternating electric field and eo is the dielectric constant (8.85*10-12 F/m).
Ionic conductivity results
from the presence of ionic impurities in the system (e.g. inorganic and organic
salts) and is dependent on the viscosity of the sample. If the concentration of
the ionic impurities remains constant during the cross-linking reaction, the
ionic conductivity makes an ideal parameter for following the changes in
viscosity that occur during cross- linking (1). Starting with an uncured powder
coating, the ionic conductivity increases rapidly with increasing temperature,
as the powder first sinters and then melts. With the onset of cross-linking, the
viscosity of the powder coating increases with a resulting parallel decrease in
ionic conductivity. This process continues until the reaction is complete and
the coating fully cured. The change in ionic conductivity during the isothermal
cross-linking reaction is used to determine the degree of conversion a as a
function of time.
The loss factor e" is composed of two components:
e" = s/2P f e0 + e"d where e"d is the component of the loss factor e" caused by dipole relaxation losses.If DEA measurements are
made at low frequency, under these conditions, the dipole relaxation component
e"d is negligible and the loss factor e" is de-termined completely by
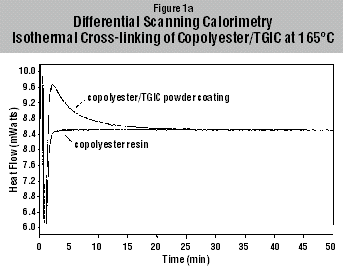
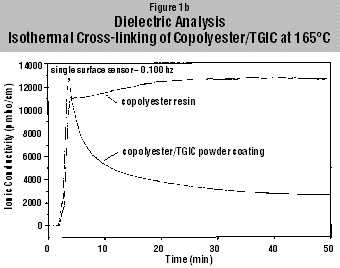
the ionic conductivity (2,3). Results of Copolyester/TGIC Cross-linking Figures
1a and 1b illustrate the isothermal cross-linking of copolyester/TGIC as
measured with DSC and with DEA at 165°C. With DSC the change in enthalpy was
recorded, whereby with DEA the change in ionic conductivity was measured.
Control measurements were made on the copolyester resin without cross-linker.
The degree of conversion measured as a function of time by DSC is given by Equation 1:
a (t) = H(t)/H0 where H(t) is the reaction enthalpy as a function of time and H0 is the total reaction enthalpy.A very good correlation between DSC and DEA is achieved if the degree of conversion measured against time by DEA is calculated using Equation 2: a (t) = {s(0)- s(t)}/{ s(0)- s(`)} where s(t), s(0) and s(`) are the corresponding values of ionic conductivity at a time (t), at the beginning of the reaction (0) and at the end of the reaction (`).
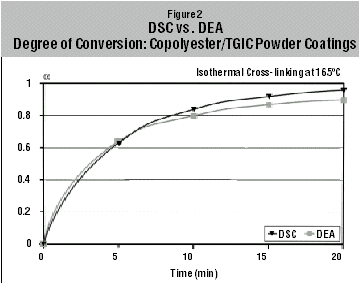
From a comparison of the two results (Figure 2), it can be seen that the two curves are very similar. Taking into account the accuracy of the two test methods, the curves can be considered virtually identical. This indicates a very good correlation between the two analytical techniques. The proposed process to determine the conversion curve of the cross-linking reaction from DEA measurements (Equation 2) assumes a linear relationship between the ionic conductivity s and the degree of conversion a.
The functional
relationship between the degree of conversion a and the ionic conductivity s is
given in the literature by Equation 3:
a = k log s + C
(where k and C are constants) (4). Very good correlation has been observed
between the DEA and DSC techniques based on a linear relationship between the
ionic conductivity and degree of conversion.
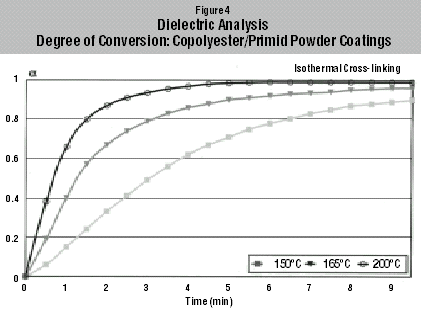
Results of Copolyester/Primid XL-552 Cross-linking
The changes in ionic conductivity with isothermal cross-linking of the Primid powder coatings at two different temperatures, 150°C and 200°C, are shown in Figure 3. The conversion curves of the Primid cross-linking reaction at three different temperatures, as calculated using Equation 2, are shown in Figure 4. At 200°C, 99% conversion is reached after 5 minutes, whereas at 150°C the conversion after 5 minutes is only 68%.The reaction profile of the powder coating cross-linking is represented by the following nth order kinetic Equation 4: da(t)/dt = k{1-a(t)} whereby, da(t)/dt is the reaction rate, n is the order of the reaction and k the appropriate rate constant. The rate constant is dependent on temperature as given by the relationship (Arrhenius Equation 5) k = A exp (-Ea/RT)
The kinetic parameters, namely the pre-exponential factor (A) and the activation energy (Ea), could be determined by plotting Ln (k) against the reciprocal of the absolute temperature (1/T).
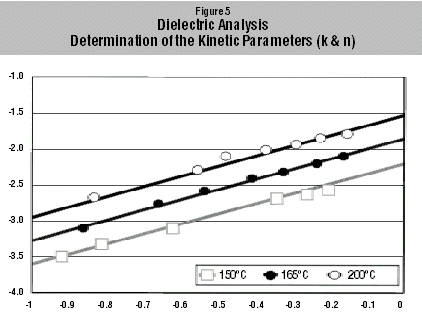
The rate constant k and the reaction order were determined by plotting log (da(t)/dt) against log (1-a(t)). The resulting curve was a straight line with a gradient n. The corresponding rate constant could then be determined by extrapolating log (1-a(t)) to zero.
The plots of log (da(t)/dt)
against log (1-a(t)) for three temperatures are illustrated in Figure 5. The
plotted values for the different temperatures lie on parallel gradients, thus
confirming the validity of the proposed kinetic equations (Equations 4 and 5).
The gradients of all three lines gave a reaction order n=1.4. Having determined
the reaction rate, both the activation energy (Ea) and the pre-exponential
factor could be estimated using the Arrhenius equation and the plot of ln k
against 1/T. The results of the kinetic analysis were as given in Table 1.
Conclusion
The reaction kinetics of polyester/Primid XL-552 were investigated by monitoring changes in ionic conductivity, with the ionic conductivity being directly influenced by the viscosity of the system. During cross-linking the viscosity of the coating increases resulting in a corresponding decrease in ionic conductivity.Comparative tests made with a polyester/TGIC powder coating using both DSC and DEA techniques resulted in a very good correlation between the two techniques. The results depicted in Figures 2 and 3 prove that DEA is a suitable method for assessing the conversion rate of polyester/Primid powder coatings.
DEA provides an accurate and reproducible method for measuring the reaction kinetics of polyester/Primid powder coatings. From the results of DEA, precise predictions about the required curing conditions (i.e. time and temperature) can be made.
Dr. Georgi Beschiaschvili is the section leader for physical chemistry, and Liselotte Tanner is the laboratory leader for physical chemistry at EMS-Chemie's Analytical Center for R&D. Manfred Wenzler is the manager of technical service at EMS-Primid.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!