Curing with Infrared and Combination Ovens
IR-enhanced convection combines the best properties of infrared and convection oven systems and produces a high-quality coating in significantly less time than is possible using convection methods alone. In addition, IR-enhanced convection produces more process-friendly characteristics than are possible with IR alone.
One limitation of IR-curing technology is its complexity. Whereas traditional coating operations require managing relatively few variables during the curing process, particularly those involving straight convection ovens, IR-enhanced convection involves more variables-but more opportunities for fine-tuning the cure. As a result, it is necessary to have a thorough understanding of the principles of both IR and convection energy transfer.
Principles of IR
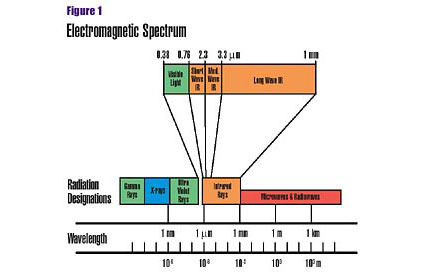
All bodies (at temperatures above absolute zero) naturally emit and absorb energy as electromagnetic waves. When a material absorbs electromagnetic energy, changes take place within the molecules. Depending on the radiation wavelength, these changes may involve electron-orbiting levels, bonds connecting atoms or the nuclei of the atoms themselves.There are three methods by which heat can be transferred from one body to another: conduction, convection and radiation. High-purity electric and gas IR operate on a principle similar to kitchen microwave ovens. IR waves and microwaves are both in the electromagnetic spectrum, which also includes radio waves, ultraviolet radiation, X-rays and visible light. The different categories of electromagnetic energy are classified by wavelength, which is the distance between radiation energy pulses (Figure 1).
Within the IR spectrum, wavelengths are divided into three bands: shortwave, which overlaps the end of the visible light spectrum (0.76 to 2.3 microns), medium wave (2.3 to 3.3 microns) and long wave (3.3 to 1,000 microns).
Trace levels of long-wavelength IR energy are emitted from most substances at room temperature. As a body warms, however, it begins to radiate increased amounts of IR energy at reduced wavelengths. When the temperature reaches approximately 1,100ºF, the emitting energy reaches the medium-wavelength IR range. Increasing the temperature even further produces short-wavelength IR energy.
When IR energy is absorbed, the bonds that join atoms within the molecule are affected. Normally the bonds vibrate with consistent amplitude. However, the amplitude increases with the absorption of energy and then returns to normal. The molecule returning to its low-energy state releases the energy as heat. It is this heating that preheats, dries or cures a coating.
Some of the IR waves intercepting a body are absorbed and converted to heat. The rest either pass through the material or are reflected back toward the energy source. If a molecule absorbed all IR energy equally, the application of IR technology would be relatively simple. However, a given type of molecule will absorb a certain wavelength of IR energy more readily than others. At some wavelengths, a large percentage of photons are absorbed while at other wavelengths, no energy may be absorbed.
The type of molecular bonds contained in a material and the vibrations they undergo determine which wavelengths of energy will be absorbed, as well as the relative strength of the absorption. Consequently, the IR-absorption characteristics of coatings tend to vary with their chemical makeup.
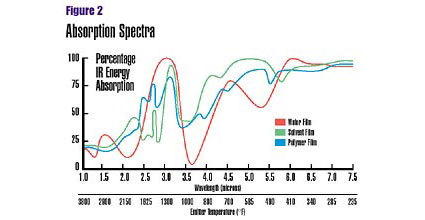
Medium-wavelength absorption highest
Most organic materials, including coatings, absorb maximum IR energy in the medium wavelength range (Figure 2). Polar groups (OH) and several free radicals, for instance, have a prominent absorption peak at approximately 3.2 microns. Typical waterborne, solventborne and powder coatings have a maximum absorption in the medium-wavelength range. The process of IR spectroscopy can determine which wavelengths a given material most readily absorbs.Once the absorption pattern has been established, the next step is to tune the IR-emitting source to the desired wavelength. This is accomplished by varying the temperature of the IR emitter. As temperature increases, the emitters radiate IR energy of increasingly shorter wavelengths.
When a coating material is exposed to a given IR wavelength, some photons will pass through the surface and into the coating itself. The rate of IR absorption in the coating will increase with the depth of penetration. Whether IR energy passes through the coating to the substrate is a function of the coating's chemistry and thickness.
High-purity IR transfer eliminates the premature hardening of coating surfaces, a problem associated with convection-oven curing and elevated levels of fugitive long-wave emissions. Skin-over, blister and pinholes are formed when heat is absorbed on the surface of the coating faster than it is absorbed within the coating. High purity eliminates these problems by reacting deeper within the coating, drying or curing the material from the inside out. This is accomplished by adjusting the IR emitters to a wavelength slightly shorter than that of the material's maximum-peak absorption wavelength. Doing so forces more of the energy to lower layers of the coating and to the coating's interface with the substrate. No premature surface hardening occurs and the result is a smooth finish.

Short-wavelength IR more sensitive
The short-wavelength band is adjacent to the visible light spectrum. This means that short-wavelength emissions are commonly accompanied by white light. Short-wavelength IR is thus more sensitive to color and reflectivity and will react differently based on the color of coatings.Since coating-film absorption for short-wavelength IR is relatively low, most of the IR radiation that is not reflected passes through to the substrate. Nevertheless, short-wavelength IR energy levels are high enough that the coating-film temperature increases rapidly. As a result, the window for proper coating absorption for some coating types is much smaller than for medium-wavelength IR, increasing the probability of overheating both the coating and the substrate. However, there are some applications where short wavelength IR is appropriate, including preheating of nonreflective surfaces.
The fine-tuning of IR emissions is effective only if the system is designed to deliver high-purity IR emissions that do not substantially alter the radiated wavelength. High-purity electric-powered IR emitters typically consist of quartz tubes coated on one side with a highly reflective material, usually gold. This gold-coated quartz serves as an envelope to minimize convective losses from the emitter coil and also serves as a primary reflector, directing maximum energy toward the target.
Gold is widely used because it reflects approximately 98% of incident IR energy. This efficient radiation property minimizes reflector surface heat-up, which would constitute energy loss. Oven walls are generally aluminum surfaces, which provide approximately 80% reflectivity. In certain applications, such as automotive waterborne finishing, oven walls are coated with gold, transforming them into secondary reflectors to improve the system's quality and energy efficiency.
An example of a high-purity, gas-powered IR emitter source would be the low-mass, ceramic matrix type, which utilizes a low-pressure, direct-fired emitter bed. Due to the nature of direct-fired combustion, this technology is not suitable for highly combustible solventborne liquid coatings.
Additionally, since it is not economically feasible for gas IR emitters to utilize quartz "windows" to minimize convective losses, the use of these high-purity gas-IR emitters in combination with direct high-velocity convection must be more segregated and limited to preheating of the coated surfaces prior to entering the high-velocity area of the convection exposure. Typically, this type of "high-purity" gas-IR emitter can be most effective when located within the "quiet" section of a convection oven found at the oven's entry.
Some systems employ lower-purity IR emitters that use primary electric emitter coils or gas-burner ports embedded in nonreflective materials, such as ceramics, or are housed in metal sheaths or castings. These absorb much of the energy and refract (radiate) it at altered longer wavelengths or convert it to convective heat. Significantly less energy is radiated within the key medium-wavelength band, and control over specific wavelength emissions is lost.
With these lower-purity IR emitters, only 20 to 30% of each kW (or Btu) of electric power is converted to IR energy within the desired absorption range of 2.2 to 3.2 microns. The balance is converted into undesirable long-wavelength IR or convective energy, which may not be able to be used in the process and must be exhausted. Using high-purity systems yields 60 to 65% energy conversions into the desired range, while generating a minimum of wasted (or even damaging) fugitive long-wavelength emissions and convective losses.

Principles of convection curing
Convection oven systems use moving air as the medium for heat transfer. This method of energy transfer is acceptable for a slow cure, but does not lend itself to accelerated cure times if high quality is to be maintained. While a wide range of temperatures and air speeds are theoretically possible, precise control over an accelerated cure is difficult.Cure times may be lowered by increasing temperature and/or the velocity of air within the oven, but the quality of the coating finish may be compromised. For example, if increasing the cure time causes the temperature to become too high, the surface of the coating may absorb more energy than it can transfer to lower layers of the coating. The result is that a skin forms on the surface, trapping water and/or solvent beneath the surface and causing blistering or pinholes.
Typical low-velocity convection ovens have air speeds of 200 fpm, while high-velocity nozzles produce nominal air speeds of up to 3,000 fpm. When high air speeds are employed to reduce cure time, the likelihood of dust and particle contamination increases. In addition, some liquid coatings cannot withstand the air-scrubbing of the surface, and powder coatings are subject to powder blow-off.
The use of indirect-fired convection heating eliminates the potential for contamination of certain sensitive coatings by combustion byproducts and/or accumulation of compounds released by the coating during thermal processing. In such a system, heat exchangers, in-line filtration and fans keep the curing environment separate from the source of heat. However, quality problems associated with high air speeds are not alleviated by the use of indirect-fired convection.
Compared to IR energy, convection curing does enjoy some advantages in some specific applications. Unlike IR, which requires line-of-sight transmission of energy to surfaces, the use of circulating air allows heat to reach areas such as interiors of 3-D shapes that are hidden from direct exposure and enables the oven to maintain all surfaces at a constant temperature.
In addition, while convection-energy transfer is slower than IR, it is more forgiving for heating substrates that vary in mass and/or thickness, such as irregular shapes. With IR-energy transfer, the longer an object is exposed, the hotter the thinner sections become due to low mass-to-surface-area ratios.
IR-enhanced convection
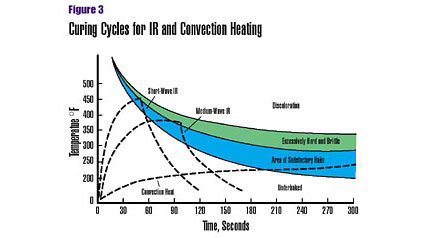
Combining the best characteristics of two somewhat dissimilar energy transfer processes-infrared and convection-produces a curing system that provides rapid, controlled energy transfer from source to coating and substrate (Figure 3). Such a system accommodates both flat surfaces and complex 3-D shapes. It can be fine-tuned to achieve a high-quality preheating and cure with a wide variety of coatings, including environmentally friendly thermoset and UV-curable powders, and waterborne liquids. And, IR-enhanced convection curing can produce productivity gains without increasing floor space.An IR-enhanced convection system begins with a "preheat" zone employing low air velocity with a series of high-purity IR emitters (electric or gas), whose energy output provides rapid temperature ramp-up of the coating and substrate.
An "equalization" zone that combines IR (electric only) and higher-velocity convection energy transfer within the same chamber follows the preheat section of the oven. This zone raises the temperature of surfaces that have a different thickness and mass or that are hidden from the line-of-sight IR energy to equalize in temperature those surfaces that were elevated in the preheat zone.
The third section, a "holding" zone, consists only of convection heating, which maintains all surfaces at a predetermined temperature. This assures a proper cure and an even finish when substrates are of uneven density or thickness.
An infinite variety of cure profiles is possible by adjusting the IR emitters' wavelength and watt density, adjusting the temperatures within the equalizing and hold zones and varying the line speed. These adjustments can be made manually or through computer controller presettings.
For powder coatings, IR-enhanced convection curing systems produce a high-quality finish in less time than is possible with either IR or convection when used alone. Thermal cycles of five to 10 minutes are possible for large, thick parts 1⁄4 to 3⁄8 inch thick. Curing cycles as short as three to six minutes are possible for 3-D sheet-metal parts. And a 24-gauge flat blank can be cured in as little as 30 to 90 seconds.
Very heavy steel parts 5 to 8 inches thick have been fully cured with a powder finish in less than 50 minutes, whereas a convection-only system would require more than two and a half hours. Actual cure times depend on the chemistry of the coating, the parts' mass-to-surface ratio and oven temperature.
Increasing the productivity of a standard convection system requires extending the oven. An IR-enhanced system, on the other hand, delivers faster cycle times in approximately the same floor space, allowing more productivity in the same space. Manufacturers can convert to new coating technologies and comply with new regulations governing solvents without expanding ovens.
High-purity, IR-enhanced convection systems help provide solutions to environmental and productivity challenges for many industries, including appliances, automotive and telecommunications. The ability to provide a high-quality finish with shorter cycle times is contributing to the growing popularity of new applications, such as postforming of precoated blanks, as well as traditional coating applications.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



