1K Polyurethane Dispersion for Conventional 2K Applications

Polyurethanes are a unique class of polymers due to their combination of both hard and soft segment blocks within the same polymer chain. This combination results in polyurethanes being able to demonstrate high surface hardness, very good chemical resistance and toughness along with excellent flexibility, particularly at low temperatures where other polymers may become brittle.1-3 Moreover, this combination of polymer characteristics leads to polyurethanes displaying excellent durability and abrasion resistance. The hard segment of the polymer, made up of urethane and urea linkages, provides the hardness and chemical resistance through the formation of strong hydrogen bonds; the hydrogen bonding can be considered a weak form of self-crosslinking (compared to covalent bond type crosslinking). The soft segment, determined by the polyol from which the polyurethane is made from, contributes to the low temperature flexibility, impact resistance and, to a large extent, the film forming capabilities of the coatings. This combination of properties has led to polyurethanes being the polymer of choice for wood floor coating applications, particularly for on-site application or refinish. Initially, these coatings were solvent, solution-based polyurethanes, however, with government regulations limiting the coating VOC, waterborne polyurethanes have gained an ever-increasing market share, particularly as their performance has been demonstrated to be comparable to or better than their solventborne counterparts in many applications.
Waterborne polymers are more user friendly than their typical solventborne counterpart and provide reduced flammability, health and odor concerns. Despite the excellent properties exhibited by polyurethanes they typically need to have some degree of crosslinking to provide the required performance needed for certain applications such as wood floor coatings. The required crosslinking is often conventionally done by the introduction of an added crosslinking component to the polyurethane. This leads to concerns on handling toxic crosslinkers, limited pot life of the mixture, the need to mix components prior to application, and the potential for waste due to exceeded pot life or mixing mistakes. This paper discusses furthering the inherent advantages of waterborne polyurethanes including their user friendliness by way of a newly developed one-component (1K) self-crosslinking polyurethane technology that performs on par with conventional commercial two-component (2K) waterborne PUDs for wood floor coatings.

Waterborne Polyurethanes
2K CrosslinkersThere are several options available to formulators for improving the performance of waterborne thermoplastic polyurethanes by crosslinking of the polymer. Among these, polyaziridines and polyisocyanates have found significant commercial application in contractor-applied wood floor coatings, mainly due to their cure effectiveness at ambient conditions in a reasonable length of time. Polyaziridines can give very effective crosslinking in carboxylic acid functionalized polyurethane dispersions with good property development after as little as 1 day curing time at room temperature. Moreover, they are readily incorporated into waterborne polymers due to their high degree of water solubility and low viscosity. Polyaziridines have a reasonably long pot life of at least one working day, or 8 hours, and purportedly can be “re-inoculated” with a second dose after the recommended pot life has expired. But polyaziridines have issues with skin irritation and are a suspected carcinogen/mutagen primarily based on the fact that they give a positive result in the Ames bacterial assay test. The use of water-dispersible poly-isocyanates as a crosslinking component of 2K PUD for floor coating applications is a somewhat newer technology that is growing in popularity. It has gained some favor over polyaziridine crosslinkers due to reduced toxicological concerns, however, they are still of concern for allergic reaction, skin irritation and asthma.4
Polyisocyanate crosslinkers can react with active hydrogen groups, such as hydroxyl groups and primary or secondary amines, on the polymer backbone or at the end of the polyurethane chains. They can also provide good properties with PUDs that do not carry significant crosslinking functionality by self-condensation via reaction with water followed by decomposition of the carbamic acid generated, releasing CO2 and generating an amine that quickly reacts with any available isocyanate to generate a urea linkage. It is generally believed that for room temperature 2K polyisocyanate crosslinked PUDs this self-condensation is the primary reaction resulting in an IPN.5 Data we have gathered (discussed later) suggest that polyisocyanate crosslinkers can provide improved durability and can give close to the same resistance properties as polyaziridine-crosslinked polyurethanes but require longer cure times to achieve them. However, water-dispersible polyisocyanates are know to be harder to incorporate into waterborne dispersions due to their higher degree of hydrophobicity and higher viscosity, usually requiring them to be cut with solvent and the need to use proper mixing. Water-dispersible polyisocyanates also have a shorter pot life than polyaziridines – closer to ~4 h for optimal performance – after which time the resulting dispersion becomes unusable either due to unacceptable performance, appearance or application properties. Furthermore, the above-described reaction of the poly-isocyanate with water can result in foam generation and entrapment in the finished coating.
Self-Crosslinking Chemistries
A few options exist to incorporate functional groups into a waterborne polyurethane that can crosslink at room temperature upon application and have the potential to provide a shelf stable system. The oldest self-crosslinking technology and currently the most popular means to provide a self-crosslinking polyurethane is via auto-oxidation of an unsaturated functionality. The unsaturated functional groups are obtained from fatty acids or natural oils high in unsaturation such as linseed, sunflower, soybean, tung and safflower oils. The auto-oxidative crosslinking reaction is fairly complicated and goes through a number of intermediates and leads to a large number of final chemical structures. An abbreviated scheme is shown in Figure 1 with the main final product believed to be the coupling of free radical oxygen atoms.6, 7 To be effective, oxidative curing requires the addition of a drier or catalyst to push the cure. These driers tend to be based on heavy metals that can be toxic, highly colored, and difficult to incorporate in waterborne polymers and easily affected by other components in the waterborne polymer or in the wood, such as tannins.
The crosslinking can be very effective but is regarded as somewhat slow unless high amounts of drier are used with a high content of “fast-drying” unsaturated groups, which can give the final dispersions and coatings a high degree of color.6-8 Moreover, the side-chain unsaturated functional groups attached to the polymer backbone are typically C16-based esters, which make the coatings much more brittle than typical polyurethanes due to less-effective polymer alignment and packing. This is analogous to the effect of the side chains in poly(meth)acrylate type polymers.
Carbonyl-amine crosslinking has also seen significant use in waterborne polymer crosslinking and can provide significant improvement in resistance properties of polyurethanes, however, it has been noted that the chemistry suffers from sub-par water resistance in the cured coatings.8 The reaction of carbonyls with amines is triggered by a drop in pH and loss of water; an abbreviated depiction of the reaction is also shown in Figure 1 and involves several intermediates (not depicted) to get to the final imine or azomethine product.
A third option for crosslinking waterborne polymers depicted in Figure 1 is through acetoacetoxy functionality either by enamine formation via reaction with polyamines or Michael reaction by nucleophilic addition to a Michael acceptor such as an acrylate group. These reactions can be a rapid and very effective means of crosslinking, however, issues revolve around hydrolytic stability of the ester, premature crosslinking and the potential to obtain undesirable color in the dispersion and applied coating.9, 10 The formation of the chromophore responsible for the color is likely related to the reaction of various amines with the acetoacetoxy groups,10, 11 some of which are typically present in polyurethane synthesis.
Experimental
FormulationThe Lubrizol 1K self-crosslinking (SCL) PUD was formulated with 13.7% diproplylene glycol n-butyl ether versus polymer solids and reduced to 30% solids; the final dispersion has a VOC of ~269 g/L and is a film former at 4 °C on uncoated paper (porous substrate). The commercial 2K PUDs were purchased from a local contactor supply outlet and used as is; the containers indicated the VOCs were <350g/L.
Chemical Resistance
Testing followed ASTM method D 1308. Coatings are tested by applying three coats of similar weight to maple veneer panels that are cured for the designated time (either 1 or 7 days) at ambient conditions before testing. One-hour spot tests are run on the surface of the finish. Sections of cotton facial pads are soaked in the test agent on the surface of the finish. After removal of the pad and test agent, the surface of the coating is blotted dry and observed for damage. The tested surface is re-evaluated after a one-hour recovery. Ratings are: 5 = no effect on finish; 4 = finish is slightly swelled or surface of finish is permanently changed; 3 = finish surface is changed and wood color has been impacted; 2 = more severe damage than 3; 1 = very severe damage to film or discoloration of wood; and 0 = finish is removed from the substrate.
Tests for specific applications or customer end uses may dictate longer exposure times. Chemical resistance evaluations run for longer than an hour are covered, using either watch glasses or a similar device to keep the test agent from evaporating. The rating system used above is also used for this testing. Chemical test agents that are typically evaluated for longer exposure times are: water, water/alcohol combinations, red wine, coffee, hand creams, and strong acids and bases.
Black Heel Mark, Mar and Scuff Resistance
The apparatus consists of a pivoting pendulum arm with a 2-lb weighted piece of hard, black rubber (such as that of a hockey puck) at the end meant to simulate a hard shoe sole capable of producing black heel markings. This is the striking surface to the test panel. The total length of the pendulum device from the arm pivot to the striking surface is ~38”. The test consists of striking the coated maple panel six times on different areas and direction on the panel with the pendulum black heel marking device. Striking is done by raising the pendulum arm to an angle of 90 – 94° of the coated panel, which is placed on the floor, and then releasing the arm under the force of gravity to strike the coated panel at the designated area. Results are expressed by visual assessment of the panel (scratching, scuffing, and black markings/discoloration) and gloss measurements. The test was evaluated and supported by statistical experimental analysis to other methods for predicting real floor wear resistance. Coating test samples were prepared as above for chemical resistance testing.
Abrasion Resistance
Testing was conducted as per ASTM D 4060 on wood panels. Pairs of panels are finished with three coats of the test subject and aged seven days at ambient conditions. Actual Taber testing is performed in a climate-controlled environment (70 °F/50% RH). Test panels are conditioned to this environment for 16 to 24 hours before testing. Taber test is performed using CS17 wheels. Each test panel is weighed initially and after each 250 cycles on a scale that measures to four units to the right of the decimal point. Each panel is run for a total of 1000 cycles. Taber abrasion values are expressed as milligrams weight loss per 1000 cycles (averaged over the pair of panels).
Scratch Resistance
The test coatings are prepared by making 6-mil draw-downs on Mylar sheets (typically scrub charts). The scratch resistance of the coatings is determined by subjecting the coating to 200 double rubs with a Scotch-Brite sponge weighted with 350 g. The sponge is rotated by 180º after 100 double rubs to attain a more uniform scratch/abrasion. The coatings are analyzed for final gloss at 20, 60 and 85 and compared to the starting gloss at the same area. Results are reported as percent gloss retention.
Test Floor Evaluation
Each coating to be tested is prepared by applying three coats of similar weight to four separate oak panels that are cured for 7 days at ambient conditions before assembly into the test floor. The test floor evaluates up to 24 coatings at one time. There are four statistically determined varied positions for each test coating that allows for equal foot traffic and wear patterns for each coating and statistical analysis of the data. Five gloss readings are taken of each coating at each position on the test floor. 60° gloss readings are taken initially when the floor is installed and once a month after that. A template is used for placing the gloss meter on the test positions to ensure that the same location is read each time a reading is taken. Monthly readings are usually discontinued after about five month’s usage. Most gloss loss occurs in the first two months of usage. About one to two thousand people cross this floor every workday. Each coating at each location on the floor is also monitored for black heel marking and other damage that may occur to the finish (high heel indentions as an example).
Solvent Swell
Data generated for solvent swells were based on dispersions at ~30% solids drawn-down 10-mil wet on Mylar film that was pre-coated with a Teflon release coating. The resulting films (~2-mils dry) are cured for a given time period (1 day or 7 days) at ambient conditions, after which time a 25-mm diameter circular film is punched out using a die. The 25-mm circular film sample is then submerged in the solvent being tested in a small glass jar for a 24-hour time period before being tested for swell. Solvent swell was determined by measuring the diameter of the swollen film in a petri dish containing some of the solvent using digital calipers and double checked with a standard metric ruler.

Discussion of Results
Chemical ResistanceFor adequate protection against commonly used cleaners, food stuff and alcohol-based materials, floor coatings need to have excellent resistance properties in order to prevent unsightly stains and blemishes to the coating as well as the substrate. A number of materials expected to be potentially encountered in residential and commercial applications that are known to be aggressive toward coatings were evaluated on the self-crosslinking PUD and compared against two commercial 2K-crosslinked controls based on polyaziridine and polyisocyanate crosslinkers. The exposure time of the coatings to the substance is given in parentheses next to the substance. The results shown in Table 1 illustrate the SCL PUD performs favorably compared to the commercial 2K controls in the gloss compositions that were tested. In particular, the performance of the 1K PUD is particularly impressive toward alcohol resistance where common thermoplastic PUDs tend to be weak. Moreover, the water resistance is excellent for the 1K SCL PUD, which was found to be a weakness for ketone-amine self-crosslinked PUDs.8

Another way to look at how effective crosslinking is in a polymer based on an objective measurement is through solvent swell experiments. The main drawback is that they do not take into consideration the adhesion or discoloration of the coating on a wood substrate being subjected to chemical attack. As mentioned in the experimental section, cured films of known dimension are submerged in the swelling solvent, and the maximum degree that the film swells is recorded. The degree of swelling is related in large part to the degree of crosslinking particularly for highly aggressive solvents. The swelled films are measured and compared to the starting film size; the lower the swell the more effective the crosslinking and solvent resistance. Several solvents were examined that are typically aggressive toward PUD coatings to determine consistency of results.
We thought it also might be useful to determine cure speed by looking at the effect of cure time (1 day vs. 7 days) at ambient conditions on solvent swell results. The results, in Table 2, show that the 1K SCL PUD performs very well in comparison to the commercial 2K-crosslinked PUD controls. The large difference for the aziridine-crosslinked PUD in NMP might be related to its crosslinking through the acid groups on the polymer. As expected, the 2K polyisocyanate-crosslinked PUD gave the most significant improvement in crosslinked performance after 1-week cure time, particularly with respect to NMP swell. However, the difference in 1-day vs. 7-day cure is not as large as anti-cipated for the 2K polyisocyanate-cured PUD, if the cure is largely self-condensation after reaction with moisture and suggests that much of the cure is complete early on. The SCL PUD did not show any additional improvement in solvent swell after the 1-day cure time, suggesting a fast crosslinking reaction.

One of the most important performance aspects of wood floor coatings is their ability to resist against aesthetically unpleasing marks from shoe soles, often termed black heel mark resistance or scuff resistance. Along with scratching or abrasion, shoe scuffing represents the main modes of floor failure in terms of change in gloss and aesthetics. If a coating does not develop strong resistance against shoe sole scuffing in a relatively short period of time, a beautifully finished wood floor can quickly start to look unsightly. This is particularly true in commercial settings such as clothing stores, which can not stay closed for extended time periods (for floor finishing), and undergo heavy customer traffic wearing a variety of shoes. The pendulum black heel mark (BHM) and scuffing device test described above was used to test the SCL PUD against popular contractor-applied 2K PUD wood floor finishes. This test is deemed one of the better “bench top” tests for determining actual real world floor coating performance based on a statistical analysis in comparison to other potential lab test methods available.



Another critical performance parameter for wood floor coatings is their ability to withstand abrasion. As mentioned previously, polyurethanes in general have very good abrasion resistance, and crosslinking likely improves the coating performance toward resistance against chemicals and scuffing or marring. However, upon crosslinking the coating system still needs to retain or show improved abrasion resistance and the ability to withstand scratching that would be experienced by a floor coating, particularly in a commercial setting where shoe soles can carry abrasive materials. Figure 5 shows the data for both gloss and satin formulas of the Lubrizol SCL PUD in comparison to the commercial 2K floor coatings using either a polyaziridine or polyisocyanate crosslinker. The data was examined both from a mg coating loss per 1000 cycles and as a function of the amount of cycles necessary to obtain wear through. In both cases the 1K SCL PUD proved superior to both of the 2K commercial finishes with the polyisocyanate being the next best and the polyaziridine being the worst for abrasion.


Evaluation of the 1K SCL PUD versus the 2K-crosslinked commercial controls by an MFMA-approved independent testing lab for wear performance supported our findings. All of the coatings tested using the “Snell accelerated soiling capsule” (MFMA specification – group 5) found them to rate excellent; an excellent rating is when the coating exhibits 0.7% +/- 0.34% or less of surface area that shows markings. The Taber abrasion test results obtained from the independent test lab exhibited a similar trend to our results; however, the differences are smaller between the 1K SCL PUD versus the 2K polyisocyanate-cured commercial PUD coating at 20 mg and 22 mg loss respectively after 1000 cycles. The 2K polyaziridine-crosslinked PUD gave a 48 mg loss.
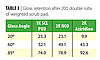
Another procedure for testing coatings in an accelerated manner to determine actual floor wear performance is a scrub pad scratch test, which looks at gloss retention after a given number of double rubs using a weighted Scoth-BriteTM scrub pad. We evaluated the same 1K SCL PUD versus the commercial 2K aziridine- and isocyanate-crosslinked PUDs in a gloss formulation as were tested in the real floor test described above for gloss retention in the scratch test. Interestingly, a similar pattern of performance was seen as in the above test floor evaluation. The results for gloss retention of the coatings after 200 double rubs of the weighted scrub pad are shown in Table 3. The 1K SCL PUD performed as well or better at the lower angle gloss retention. Oddly, the 2K aziridine PUD coating that performed the worst at the low angle gave the best gloss retention at the very high angle of 85 degrees.
Summary
Lubrizol has developed a new self-crosslinking polyurethane dispersion technology that has demonstrated performance comparable to that of commercial 2K contractor-applied wood floor finishes. Performance of the new 1K PUD was evaluated in terms of chemical, scuff or black heel mark, scratch and abrasion resistance against commercial 2K-crosslinked waterborne polyurethane coatings along with current commercial 1K PUD floor coatings. Furthermore, statistically designed test floor data along with outside lab MFMA performance specifications qualification data supports the lab bench top test results that the performance of the Lubrizol 1K PUD is on par with commercial 2K PUD wood floor coatings. The advantages of the new self-crosslinking 1K PUD are it’s user friendliness, which eliminates potential for waste and performance issues resulting from poor mixing, limited pot life, and health issues related to handling the crosslinking component. Combined with the fact that the new 1K SCL PUD is easy to apply and quick to dry, this can save time and money for application of the coating at a wood floor coating job site.Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







