Highly Weather-Resistant Radiation-Curable Monomers and Oligomers for Exterior Applications
The
weathering resistance of an exterior coating is one of the main properties
required by the end user. To achieve this goal, careful selection of raw
materials that comprise the final composition is critical. The backbone
structures of several monomers and oligomers have been investigated, subjected
to accelerated outdoor weathering testing (Q-TRAC) and correlated with actual
Florida exposure. The results were also correlated with data attained from
accelerated laboratory testing.
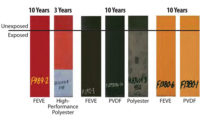
The performance of these products has also been compared to a standard two-component (2K) urethane intended for automotive topcoat applications. This ongoing study has shown that these acrylates, which are free from hindered amine light stabilizers (HALS) and light absorbers, can withstand greater than three years of Florida exposure without degradation. These tests are ongoing and will continue for at least five years. Since the testing was conducted in accordance with automotive test standards, these materials are suitable for any application requiring superior outdoor durability.
Along with reporting results of the outdoor weathering study, a correlation is provided comparing the different types of accelerated weathering laboratory equipment in terms of severity and harshness as they relate to outdoor testing in Florida and Arizona. Other areas of importance explored include:
• Backbone structure of monomers and oligomers that result in a high level of weathering resistance;
• The importance of photoinitiator selection on yellowing resistance;
• The impact of HALS and UV light absorbers to enhance yellowing resistance and film degradation.

 Photo degradation, or photolysis, also contributes to
polymer deterioration. Photolysis relates to absorption of light by a polymer
chromophore. This results in an energy transfer reaction leading to film
degradation. Aromatic polymers are inherently not stable and degrade rapidly
during exterior exposure. When toluene diisocyanate (TDI) or MDI are used in
the urethane polymer reaction, severe yellowing results from chain scission and
photo degradation (Figure 2).
Photo degradation, or photolysis, also contributes to
polymer deterioration. Photolysis relates to absorption of light by a polymer
chromophore. This results in an energy transfer reaction leading to film
degradation. Aromatic polymers are inherently not stable and degrade rapidly
during exterior exposure. When toluene diisocyanate (TDI) or MDI are used in
the urethane polymer reaction, severe yellowing results from chain scission and
photo degradation (Figure 2).

On the other hand, UV-curable coatings are one-component systems that do not require metering or mixing, do not contain solvents, do not require heat, and curing is nearly instantaneous. In addition they typically have a shelf life exceeding six months, and in most cases greater than a year.
The urethane acrylate oligomers used in this study are made using aliphatic isocyanates. These are well known to have good exterior durability, as there are no chromophores that are subject to oxidation or UV degradation, resulting in cured films that will resist yellowing and micro cracking (Figure 3).
 Polyether
polyols also exhibit poor exterior durability when they are subjected to the
effects of heat and UV exposure, as they can oxidize to form hydro-peroxides
causing yellowing and chalking of the cured films. Polyester polyols however do
not degrade and are used to form the urethane linkage with the isocyanate,
which in turn are end-capped with the functional acrylate groups (Figure
4).
Polyether
polyols also exhibit poor exterior durability when they are subjected to the
effects of heat and UV exposure, as they can oxidize to form hydro-peroxides
causing yellowing and chalking of the cured films. Polyester polyols however do
not degrade and are used to form the urethane linkage with the isocyanate,
which in turn are end-capped with the functional acrylate groups (Figure
4).
 Table
1 offers a listing of the oligomers used in this study. Each of them is an
aliphatic urethane acrylate having a polyester backbone structure; the
functionality ranges from 2-6.
Table
1 offers a listing of the oligomers used in this study. Each of them is an
aliphatic urethane acrylate having a polyester backbone structure; the
functionality ranges from 2-6.
 Oligomers
are inherently of high molecular weight, thus they also tend to be high in viscosity.
To use these oligomers in 100% reactive formulations, low-viscosity functional
diluents or monomers are needed to attain a workable application viscosity for
the final formulation. Monomers must also be selected that have good
weathering-resistant properties (Figure 5). A class of monomers that fits these
criteria is materials having an alkane backbone structure. They are known for
having high heat resistance and are not subject to chromophore degradation.
They also have the added benefit of being water hating and, owing to their
linear or planar carbon ring structure, have excellent adhesion
properties.
Oligomers
are inherently of high molecular weight, thus they also tend to be high in viscosity.
To use these oligomers in 100% reactive formulations, low-viscosity functional
diluents or monomers are needed to attain a workable application viscosity for
the final formulation. Monomers must also be selected that have good
weathering-resistant properties (Figure 5). A class of monomers that fits these
criteria is materials having an alkane backbone structure. They are known for
having high heat resistance and are not subject to chromophore degradation.
They also have the added benefit of being water hating and, owing to their
linear or planar carbon ring structure, have excellent adhesion
properties.
 Table
2 provides a list of monomers used in this study and their viscosities and
surface tension.
Table
2 provides a list of monomers used in this study and their viscosities and
surface tension.

The equipment, made by the Q-Panel Company, was used to conduct the weathering tests. The following describes each of the apparatus used along with the test conditions.
A) QUV Testing- the conditions were: 8 hours of UV radiation @ 60 °C, followed by 4 hours dark condensation @ 40 °C. The lamps were replaced every 400 hours to ensure constant UV intensity. The bulb used was a UVA 340 from Q-Panel. The output is from 300 - 400 nanometers centered at 340 nm. This compares very favorably to natural sun light conditions.
B) Q-SUN Xenon Test Chamber (Model 3100) equipped with humidity control to more closely replicate the high humidity conditions seen in Florida. The cycle conditions were as follows.
1) 40 minutes of light at 70 ºC (black panel temperature) -50% relative humidity.
2) 20 minutes of light and water spray.
3) 60 minutes of light at 70 ºC (black panel temperature) -50% relative humidity.
4) 60 minutes of darkness at 38 ºC (black panel temperature) - 95% relative humidity.
C) Q-TRAC Testing – The sample to be tested is placed at the focal point of several mirrors, which collect the light and direct it to the test panel. This device then tracks the sun across the horizon, thus maximizing the sunlight exposure (Figure 6).
D) Florida Exposure- Inland, facing South at a 5 degree angle.
It was determined through empirical testing and observation that QUV testing is the harshest of the accelerated weathering methods. It is a good screening tool, as many samples can be tested at one time, and if a viable control sample is used, reliable results can be attained quickly, in less than 1000 hours of exposure. But using this method as the lone test criteria will result in coatings that are over engineered due to the severity of the test. Xenon testing is less severe than QUV testing and very closely replicates the results that are produced in a Florida test. The least severe is Q-TRAC testing, as the conditions tend to be very arid and may mask the effect of surface erosion induced by rain and high humidity.
The gloss retention and the Yellowness Index were recorded as a function of accelerated weathering exposures as well as “real time” Florida exposure. Gloss was measured at 60º by following the ASTM D 523-89 procedure and by using a micro-gloss meter from BYK-Gardner. A BYK Color Guide (geometry 45/0, illuminant/observer: D65/10) was used to measure the Yellowness Index (YI) according to ASTM E 131-98.
Physical properties of the cured films are important to the formulator for selection of the correct materials to meet the end-use application requirements. Tensile experiments were performed on an Instron 1122 apparatus according to the ASTM D 882 procedure. Sample sizes were 25 mm x 8 mm with a thickness of 100 microns. The stress-strain curves were recorded to determine the Young’s Modulus (YM), the stress and strain at break (percent elongation @ failure).
Since oligomers tend to be more flexible they were tested “neat”, while monomers can at times be too brittle to test. The monomers were added to very flexible oligomers known to have excellent weathering properties. Since these blends can be very high in viscosity they were cut with 50% acetone and applied to cold-rolled steel having an electrocoat primer and white basecoat. Unless otherwise specified, all formulations contained 3.0% of TPO photoinitiator (2,4,6-trimethylbenzoyl diphenylphosphine oxide) known for its photo-bleaching properties.
These mixtures were applied to the substrate to yield a film thickness of 35-40 microns, after the acetone was evaporated. This is the film thickness used throughout this study unless otherwise stated. A 600 w/in. “V” lamp in air followed by a 600 w/in. “H” lamp in nitrogen was used for curing. The conveyor speed was set at 10 FPM.

It is interesting to note that these oligomers are identical in backbone structure but the gloss retention is poorer and the YI higher as molecular weight of the oligomer increases. This is an indication that in order to have extended outdoor weathering properties lower molecular weight oligomers are preferred in most cases.
QUV Testing was also used to screen oligomers having very different backbone structures. These materials consisted of a Bis A epoxy acrylate (CN120), a di-functional polyester acrylate (CN2003) with and without HALS, and the “control” oligomer, (CN9001), a di-functional aliphatic polyester acrylate.
Bis A epoxies have been the workhorse oligomer for UV curing applications for many years as they are low in cost along with being very hard and abrasion resistant. A high degree of chemical resistance is also observed. Results again support that for ultimate outdoor performance aliphatic urethane acrylates are needed. However a good cost/performance compromise can be attained using polyester acrylates.
 Epoxy
acrylates are most often offered as a blend with monomers to reduce the
viscosity to a workable level. In addition, epoxy acrylates yellow quite badly,
thus are not suitable for any application where yellowing resistance is
critical. In contrast polyester acrylates are fast curing and tend to have a
much lower viscosity than Bis A type oligomers. They also inherently have
better yellowing resistance than epoxies. When HALS is added to the formulation
they can offer a reasonable degree of yellowing resistance as depicted in
Figure 8.
Epoxy
acrylates are most often offered as a blend with monomers to reduce the
viscosity to a workable level. In addition, epoxy acrylates yellow quite badly,
thus are not suitable for any application where yellowing resistance is
critical. In contrast polyester acrylates are fast curing and tend to have a
much lower viscosity than Bis A type oligomers. They also inherently have
better yellowing resistance than epoxies. When HALS is added to the formulation
they can offer a reasonable degree of yellowing resistance as depicted in
Figure 8.

Each of the test panels was exposed for a total of 4,000 hours. This is the equivalent of more than three years of Florida exposure. It is not surprising benzophenone does not resist yellowing well. TPO on the other hand has an initially high YI. But since TPO is photo-bleaching the YI drops significantly after 500 hours exposure. In contrast Esacure KIP-150 is low in YI from the start and remains so for the duration of the test. The 2K urethane has the lowest YI initially and increases slowly throughout the study. It is important to note that the UV cured chemistry outperforms the polyurethane without the aid of HALS. It also shows that PI selection is critical to attain weathering resistance.

All of the oligomers perform well with the exception of CN929 and CN9006, where a rather dramatic loss of gloss is noted. The other products are all di-functional. CN929 is tri and CN9006 is hexa-functional. With higher functionality a greater degree of crosslinking occurs resulting in more brittle films when subjected to the rigors of heat and light. CN929 and CN9006 can however be blended with more flexible materials to optimize the end use properties or they may be applied at a lower film thickness. Keep in mind that these films are in the 35-40 micron range. No evidence of cracking was observed with the higher functionality oligomers at a 5-10 micron film thickness.
 These oligomers have a wide range of physical
properties ranging from very tough and hard to very elastomeric. No single
product will offer all of the properties for a given application. Thus the
physical properties for each of the oligomers are listed so oligomers may be
blended to enhance performance (Table 4).
These oligomers have a wide range of physical
properties ranging from very tough and hard to very elastomeric. No single
product will offer all of the properties for a given application. Thus the
physical properties for each of the oligomers are listed so oligomers may be
blended to enhance performance (Table 4).
 Monomers are also an important consideration, as they
serve not only as viscosity reducers but contribute to performance properties
and enhance weathering resistance. The monomers tested are listed in Table 5.
They are all alkane-type structures known for excellent yellowing and moisture
resi stance.
Monomers are also an important consideration, as they
serve not only as viscosity reducers but contribute to performance properties
and enhance weathering resistance. The monomers tested are listed in Table 5.
They are all alkane-type structures known for excellent yellowing and moisture
resi stance.
It is noteworthy that alkoxylated monomers are to be avoided when formulating for weathering resistance. Although they do not yellow, poor gloss retention was observed as evidenced by the performance of CD564 and SR454. Being alkoxylated causes them to be less hydrophobic, making them subject to surface erosion by moisture attack.
This paper was presented at RadTech 2008, Chicago.
The performance of these products has also been compared to a standard two-component (2K) urethane intended for automotive topcoat applications. This ongoing study has shown that these acrylates, which are free from hindered amine light stabilizers (HALS) and light absorbers, can withstand greater than three years of Florida exposure without degradation. These tests are ongoing and will continue for at least five years. Since the testing was conducted in accordance with automotive test standards, these materials are suitable for any application requiring superior outdoor durability.
Along with reporting results of the outdoor weathering study, a correlation is provided comparing the different types of accelerated weathering laboratory equipment in terms of severity and harshness as they relate to outdoor testing in Florida and Arizona. Other areas of importance explored include:
• Backbone structure of monomers and oligomers that result in a high level of weathering resistance;
• The importance of photoinitiator selection on yellowing resistance;
• The impact of HALS and UV light absorbers to enhance yellowing resistance and film degradation.

Chemistry Selection
Polymers are composed of different chemical bonds that are liable to degrade over time. Environmental factors, such as heat or UV radiation, can accelerate this process. There are many different scenarios that can lead to polymer degradation and they include thermal, photo, chemical or mechanical factors. However, the most dominating mechanisms contributing to polymer degradation are oxidation and photolysis. Radicals are produced by reactions with heat and light. Once the radicals are formed, they can react with oxygen to form hydro-peroxides. At this point the process becomes self-propagating, resulting in film or coating failure (Figure 1).

Materials Tested
Acrylic urethane coatings are commonly used due to their durability and superior weathering resistance compared to other resin systems. Until now, the most common urethane coating type used in automotive applications is a solvent-based 2K system. But this coating type is under increasing pressure from regulatory agencies to limit the amount of solvents released into the environment. Another drawback of this 2K mixture is its very short pot life.On the other hand, UV-curable coatings are one-component systems that do not require metering or mixing, do not contain solvents, do not require heat, and curing is nearly instantaneous. In addition they typically have a shelf life exceeding six months, and in most cases greater than a year.
The urethane acrylate oligomers used in this study are made using aliphatic isocyanates. These are well known to have good exterior durability, as there are no chromophores that are subject to oxidation or UV degradation, resulting in cured films that will resist yellowing and micro cracking (Figure 3).





Tests Conducted
Moisture and yellowing resistance were tested using two different types of test apparatus to attain accelerated laboratory results. These included the QUV and Q-SUN Xenon test chamber. Accelerated outdoor testing was also conducted through Q-TRAC testing in Arizona and actual outdoor weathering tests are still underway in Florida. These tests were conducted in accordance with the Automotive Test Method SAE J 1961-02, which is used to qualify exterior topcoats for automotive applications. Our reasoning was that if this harsh test method could be passed then the acrylated materials would be suitable for applications with less stringent requirements.The equipment, made by the Q-Panel Company, was used to conduct the weathering tests. The following describes each of the apparatus used along with the test conditions.
A) QUV Testing- the conditions were: 8 hours of UV radiation @ 60 °C, followed by 4 hours dark condensation @ 40 °C. The lamps were replaced every 400 hours to ensure constant UV intensity. The bulb used was a UVA 340 from Q-Panel. The output is from 300 - 400 nanometers centered at 340 nm. This compares very favorably to natural sun light conditions.
B) Q-SUN Xenon Test Chamber (Model 3100) equipped with humidity control to more closely replicate the high humidity conditions seen in Florida. The cycle conditions were as follows.
1) 40 minutes of light at 70 ºC (black panel temperature) -50% relative humidity.
2) 20 minutes of light and water spray.
3) 60 minutes of light at 70 ºC (black panel temperature) -50% relative humidity.
4) 60 minutes of darkness at 38 ºC (black panel temperature) - 95% relative humidity.
C) Q-TRAC Testing – The sample to be tested is placed at the focal point of several mirrors, which collect the light and direct it to the test panel. This device then tracks the sun across the horizon, thus maximizing the sunlight exposure (Figure 6).
D) Florida Exposure- Inland, facing South at a 5 degree angle.
It was determined through empirical testing and observation that QUV testing is the harshest of the accelerated weathering methods. It is a good screening tool, as many samples can be tested at one time, and if a viable control sample is used, reliable results can be attained quickly, in less than 1000 hours of exposure. But using this method as the lone test criteria will result in coatings that are over engineered due to the severity of the test. Xenon testing is less severe than QUV testing and very closely replicates the results that are produced in a Florida test. The least severe is Q-TRAC testing, as the conditions tend to be very arid and may mask the effect of surface erosion induced by rain and high humidity.
Parameters Measured
The main criteria for monomers and oligomers that are intended for outdoor use is the ability to resist yellowing and chalking when exposed to heat and light radiation. Also, they must be impervious to surface erosion through moisture exposure. This is typically evidenced by loss of surface gloss.The gloss retention and the Yellowness Index were recorded as a function of accelerated weathering exposures as well as “real time” Florida exposure. Gloss was measured at 60º by following the ASTM D 523-89 procedure and by using a micro-gloss meter from BYK-Gardner. A BYK Color Guide (geometry 45/0, illuminant/observer: D65/10) was used to measure the Yellowness Index (YI) according to ASTM E 131-98.
Physical properties of the cured films are important to the formulator for selection of the correct materials to meet the end-use application requirements. Tensile experiments were performed on an Instron 1122 apparatus according to the ASTM D 882 procedure. Sample sizes were 25 mm x 8 mm with a thickness of 100 microns. The stress-strain curves were recorded to determine the Young’s Modulus (YM), the stress and strain at break (percent elongation @ failure).
Since oligomers tend to be more flexible they were tested “neat”, while monomers can at times be too brittle to test. The monomers were added to very flexible oligomers known to have excellent weathering properties. Since these blends can be very high in viscosity they were cut with 50% acetone and applied to cold-rolled steel having an electrocoat primer and white basecoat. Unless otherwise specified, all formulations contained 3.0% of TPO photoinitiator (2,4,6-trimethylbenzoyl diphenylphosphine oxide) known for its photo-bleaching properties.
These mixtures were applied to the substrate to yield a film thickness of 35-40 microns, after the acetone was evaporated. This is the film thickness used throughout this study unless otherwise stated. A 600 w/in. “V” lamp in air followed by a 600 w/in. “H” lamp in nitrogen was used for curing. The conveyor speed was set at 10 FPM.

QUV Testing
As mentioned, this device is an excellent screening tool that allows for rapid screening of monomer and oligomer variables. Early in this study this equipment was used to optimize the weathering performance of materials prior to embarking on longer-term testing. One of the parameters studied was the effect of molecular weight of the oligomer and its influence on gloss retention and YI (Figure 7).It is interesting to note that these oligomers are identical in backbone structure but the gloss retention is poorer and the YI higher as molecular weight of the oligomer increases. This is an indication that in order to have extended outdoor weathering properties lower molecular weight oligomers are preferred in most cases.
QUV Testing was also used to screen oligomers having very different backbone structures. These materials consisted of a Bis A epoxy acrylate (CN120), a di-functional polyester acrylate (CN2003) with and without HALS, and the “control” oligomer, (CN9001), a di-functional aliphatic polyester acrylate.
Bis A epoxies have been the workhorse oligomer for UV curing applications for many years as they are low in cost along with being very hard and abrasion resistant. A high degree of chemical resistance is also observed. Results again support that for ultimate outdoor performance aliphatic urethane acrylates are needed. However a good cost/performance compromise can be attained using polyester acrylates.


Q-SUN, Testing (Xenon Test Chamber)
The Q-SUN, Model 3100, was used to examine the effects of differing PIs on yellowing resistance when blended with the control oligomer, CN9001. All PIs were added at a 3.0% level. These results were compared to a 2K polyurethane commonly used for automotive topcoats. The PIs tested were benzophenone, TPO and a polymeric alpha hydroxyl ketone type initiator, Esacure® KIP-150. The results are presented in Figure 9.Each of the test panels was exposed for a total of 4,000 hours. This is the equivalent of more than three years of Florida exposure. It is not surprising benzophenone does not resist yellowing well. TPO on the other hand has an initially high YI. But since TPO is photo-bleaching the YI drops significantly after 500 hours exposure. In contrast Esacure KIP-150 is low in YI from the start and remains so for the duration of the test. The 2K urethane has the lowest YI initially and increases slowly throughout the study. It is important to note that the UV cured chemistry outperforms the polyurethane without the aid of HALS. It also shows that PI selection is critical to attain weathering resistance.

Florida Weathering Results
Accelerated laboratory tests are useful for performance optimization of the chemistry relatively quickly. However there is no substitute, especially when it comes to automotive applications for, real time Florida exposure. After much lab testing and reformulation a series of oligomers and monomers were selected for testing. The oligomers were tested “neat” while the monomers were added to CN9001 at a 50% level. Three replicate panels were prepared for each chemistry variable. No HALS or light absorbers were added. Table 3 lists the oligomers tested along with YI and gloss retention data initially and after an exposure of 900 Mega-joules/sq. meter Total Ultraviolet Radiation (TUVR). Assuming there is 280 MJ/sq. m TUVR in an average year in Florida this represents 3.2 years of Florida testing.All of the oligomers perform well with the exception of CN929 and CN9006, where a rather dramatic loss of gloss is noted. The other products are all di-functional. CN929 is tri and CN9006 is hexa-functional. With higher functionality a greater degree of crosslinking occurs resulting in more brittle films when subjected to the rigors of heat and light. CN929 and CN9006 can however be blended with more flexible materials to optimize the end use properties or they may be applied at a lower film thickness. Keep in mind that these films are in the 35-40 micron range. No evidence of cracking was observed with the higher functionality oligomers at a 5-10 micron film thickness.


It is noteworthy that alkoxylated monomers are to be avoided when formulating for weathering resistance. Although they do not yellow, poor gloss retention was observed as evidenced by the performance of CD564 and SR454. Being alkoxylated causes them to be less hydrophobic, making them subject to surface erosion by moisture attack.
Conclusion/Observations
When prudent choices are made in chemical backbone, structure and functionality, coatings formulated for and cured by ultraviolet energy can offer many benefits over 2K polyurethane chemistry. They can be processed quicker, offer extended shelf life and are safer to handle. In addition, there are no emissions to pollute the atmosphere, and they can outperform 2K systems without the use of HALS or light absorbers. Although initially developed and tested according to strict automotive standards, the materials highlighted in this study are suitable for any end use requiring superior outdoor performance. nThis paper was presented at RadTech 2008, Chicago.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!