UV Stabilization of Waterborne Coatings

Besides high efficiency, UV stabilizers have to meet some other basic, but most important requirements. To grant such high efficiency, perfect compatibility, that is excellent solubility in the film-forming binder, is a definite must. This requires high permanence and low volatility as well, especially with stoving enamels. These properties must be present along with no negative side effects such as color contribution, interaction with other paint elements or influence on crosslinking.
For obvious reasons, UV stabilizers must be water-repellant. Precipitation, condensation, humidity or other water conditions would easily extract the stabilizers from the film. But such necessarily hydrophobic UV stabilizers are often rather tricky to incorporate into aqueous systems.
Incorporation of Hydrophobic UV Stabilizers
The traditional method of waterborne paint manufacture offers three stages where these additives could be incorporated: (1) addition to the millbase; (2) addition with the let-down; and (3) addition as an aqueous preparation (Figure 1).
Addition to the Millbase
Although this method generally leads to easy and safe incorporation, it is certainly not recommended. The problem is the facile adsorption of UV stabilizers on the pigment surface. As a result, the additives are removed from the binder-matrix and become, at least, partially ineffective.
Addition With the Let-Down
Addition of the UV stabilizer at this point requires a distinctly prolonged agitation. However, prolonged agitation may cause air and CO2 intake, and, in extreme cases, cause coagulation of the binder. In addition, the paint chemist faces a further problem: the range of potentially suitable UV stabilizers on the market is rather limited.
Addition as a Water-Compatible Preparation
In practice, this incorporation technique is the most common, simple and safe method. Usually, the additives are pre-mixed or diluted into other suitable components of the formulation prior to addition to the batch. But that requires additional work and time. Furthermore, such a master batch is in most cases only suitable for one single paint formulation.
Diluting the hydrophobic UV stabilizers in water-miscible solvents bears another risk: the so-called ‘gold washing effect’. As soon as the solution is poured into the aqueous phase, the hydrophilic solvent is sucked off, releasing the water-repellant additive. As a result, the UV stabilizer precipitates. Quite often, that ‘trick’ leads to even more problems.
UV-Stabilizer Dispersions
Besides excellent efficiency, UV-stabilizer dispersions have to meet further requirements concerning broad compatibility; easy handling and safe stock keeping; and ecology, safety and health issues.
Enabling a broad field of application means to find the best compromise. The highest request for UV stabilizers is identified with high-end coatings, such as OEM, auto refinish, etc. In order to meet that challenge, a universal compatibility seems to be impossible.
A suitable dispersion viscosity allows its easy incorporation. The addition of a retention aid and stabilization against microbiological attack must permit a storage time of more than two years.
Hazard labeling must solely depend on the dispersed UV stabilizer. All other elements of the dispersion must not contribute to any labeling requirements. This includes VOCs as well. A general UV-stabilizer dispersion composition is shown in Table 1.
Resin- or Surfactant-Based?
Surfactant-based preparations offer a broader range of compatibility in most cases. In principle, resin-based preparations often carry a lower additive load. The decision in favor of surfactants was especially driven by aspects of broad compatibility and environmental reasons. Surfactant-based preparations are, in most cases, biodegraded more quickly.
UV Stabilizer
We found optimal conditions at a UV stabilizer load of ca. 52% and D50 at 0.5 – 2.0 µm and D90 at < 3.5 µm. Lower particle size may pander the easy incorporation, but also increases the viscosity.
Surfactant
At the given concentration and particle size distribution, we found that an optimal blend of surfactants is a mixture of anionic and non-ionic tensides at a concentration of ca. 6 – 7 %. The surfactant composition controls the stability of the preparation while the concentration controls the ease of incorporation and the general compatibility. Also, the higher the surfactant concentration, the higher the risk of undesired secondary effects.
Storage stability is imposed by the optimized blend of surfactants. The influence of temperature on storage stability is more decisive than storage time at room temperature.
Preservative
With the necessary preservation of the aqueous UV stabilizer dispersions, focus on environment, health and safety is the most important aspect, in addition to preservation efficiency. Isothiazolinone at a concentration of < 1.0% meets this requirement, since it does not contain nor release HCHO, and is free of Bronopol®.
Retention Aid
Crusting and drying of the UV stabilizer dispersion is prevented by the addition of a suitable retention aid (maximum of 10%). The selected polyglycol also allows crosslinking of the main resin component with isocyanates and does not limit the applicability of the preparation in waterborne 2K-systems.

Compatibility of UV Stabilizers
In all cases, incorporation should be checked by a compatibility test. Pouring the thinned liquid binder composition on a glass plate and assessing the dried film is a suitable test that is simple and leads to reliable results. Specks and bits visible to the eye are unacceptable. An initial slight haze, which dissipates upon stoving or drying overnight, can usually be tolerated.
According to our findings, compatibility depends
primarily on the solubility of the diluted UV stabilizer in the diluting
medium, i.e., the binder system. Its preparation form may only influence the
incorporation in the rare case of incompatibility of surfactants from the
dispersion with other paint ingredients (Figure 2).

Radical Scavenger
Basic HALS acts as a co-neutralizer, since its amine function leads to a pkB of <5.5. Due to that fact, the aqueous preparation of the organic binder moves from the character of a dispersion toward the character of a solution And, in so doing, the molecular weight of the binder gains more influence on the viscosity. That may shorten the storage stability of the wet paint. In extreme cases the wet paint can coagulate very quickly. In such cases, non-basic HALS or HALS at higher pkB are recommended.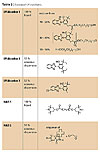
Exposure Test Results
We compared traditional and dispersed UV stabilizers (Table 2) in two different coatings: a truck coating and a wood protective varnish.
Truck Coating
A light red, aqueous 2K-PUR-acrylic monocoat was stabilized with different stabilizer packages. All relevant application parameters like film thickness, cure conditions, total concentration of stabilizers etc. were kept constant. The coating’s poor opacity required an UV absorber with the HALS.
Comparing the UV Absorbers
The molar extinction of UV absorbers of the same class is within very narrow limits, practically constant. However, due to their different molecular weights, their extinctions related to the gram differ drastically. Thus, to reach the same absorption, the concentration of UV Absorber 2 can be much lower than that of UV Absorber 1. Keeping the total concentration of all UV stabilizers constant allows a higher dosage of HALS. Figure 3 compares the absorptivity of two absorbers at the same concentrations.Accelerated weathering tests were carried out over 2,250 hours according to SAE J 1960 in a W-O-M - ASTM G 155 Cycle 7. This test is a broadly accepted accelerated exposure test in the automotive industry. Intermediate assessments were done in intervals of 250 hours. All panels were in test at the same time.
Blistering, cracking or film softening could not be detected during the exposure on any test panel.
The higher concentration of HALS explains the better gloss retention of the Stabilizer Package 2 (Figure 4). Gloss increase during the very first time of exposure is frequently observed. Migration of any paint ingredients to the coatings surface might be one potential reason for this effect.
Although the concentration of the UV absorber in Stabilizer Package 2 is lower, the color stabilization of both stabilizer packages is identical within very narrow limits (Figure 5).

Wood Protective Coating
A varnish based on an aqueous acrylic emulsion was tested according to ISO 11 341-A in a W-O-M over 2,000 hours. Intermediate assessments were done in intervals of 500 hours. All panels were in test at the same time.The different UV absorbers were added at a rate of 1.0% related to delivery form on solid resin. The film thickness was 60 ± 5 µm in all cases.
The superior performance of UV Absorber 2 against UV Absorber 1 is again related to the lower molecular weight and, thus, higher UV absorptivity; the excellent performance of UV Absorber 3 is explained by its bathochromic absorption shift (Figure 6).

Summary
The stabilizing efficiency and the compatibility are determined by the use of the individual properties of the UV stabilizers, rather than their application form. Applied in waterborne coatings, pre-dispersed UV stabilizers:- complete the range of applicable UV stabilizers;
- allow easy and safe incorporation;
- can cut raw material costs; and
- can outperform traditional UV stabilizer packages
This paper was presented at The Waterborne Symposium, Advances in Intelligent Coatings Design, February, 2007 New Orleans, LA. The Symposium is sponsored by The University of Southern Mississippi, Department of Polymer Science.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



