A Green Tool for Selective Oxidation Reactions
Growing demand for clean processes has made it necessary to develop high-performance catalysts that comply with the concepts of “green chemistry” or “sustainable development” for issues such as metal substitution, energy consumption, recycling and waste reduction.
The corresponding catalyst Oxynitrox S1001 from Arkema takes these market requirements into account. This article discusses the advantages and the applications of Oxynitrox S100, following a brief review of the literature.

Background
Oxidation reactions are of great interest in fine chemistry, at both the laboratory and industrial scale. There are numerous applications using the conversion of primary alcohols into aldehydes.Two types of reactions could fit in with this scheme. First of all, there are stoichiometric reactions involving the use of strong oxidizing agents such as: the complex chromium (VI) oxide/pyridine (Collins or Sarret reagent); pyridinium chlorochromate (Corey reagent); oxalyl chloride/DMSO (Swern reagent); dimethylsulphide/N-chlorosuccinimide (Corey-Kim reagent); Dess-Martin periodinane; SO3/pyridine; KmnO4; MnO2; RuO4, etc. Secondly, there are catalytic dehydrogenation reactions with catalysts such as copper chromite, Raney nickel, palladium acetate, etc. All these reactions do not fit in perfectly with the responsible care approach, which is now a priority in all chemical reactions. In effect, they present a number of drawbacks such as a high amount of metal waste, poor selectivity, safety issues in some cases, or harsh conditions.
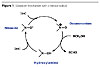
A new family of green catalysts has appeared in the last few years. TEMPO, or 2,2,6,6-tetramethyl-1-piperidinyloxy radical, is the most representative member of this family, and its efficiency in oxidation reactions is well documented.2,3 The oxidation of alcohols into aldehydes, ketones and carboxylic acids uses a catalytic amount of the nitroxyl radical and a stoichiometric amount of an oxidant such as sodium hypochlorite, m-chloroperbenzoic acid, sodium bromite, sodium chlorite, trichloroisocyanuric acid, bis(acetoxy)iodobenzene, n-chlorosuccinimide, or oxygen in combination with CuCl or RuCl2(PPh3) 3. The nitroxyl radical is converted into an active species, which is the corresponding oxoammonium ion, and is then able to oxidize various substrates (Figure 1).3
Among these substrates, alcohols are converted into aldehydes, ketones or acids; diols into lactones; sulfides into sulfoxides; benzylic ethers into esters; or a-hydroxy-lactame into anhydrides. Nevertheless, the TEMPO or hydroxyl-TEMPO structures present several drawbacks such as poor thermal stability, strong volatility with a tendency to sublimation, high solubility in water with ensuing difficulties in treating the aqueous wastes, non-negligible toxicity, and a complex synthesis route involving several reaction steps. This is the reason why Arkema decided to develop a nitroxyl catalyst, known under the tradename Oxynitrox S100.

Catalyst Characteristics
Oxynitrox S100 is a catalyst designed for oxidation reactions and is produced by an industrial process patented by Arkema.4 It is classified as a green catalyst as it does not contain any metal. Additionally, it can efficiently replace classic metal catalysts such as copper chromite, chromium derivatives, catalysts based on ruthenium, molybdenum, silver, cerium, etc. It belongs to the family of nitroxyl radicals and features an oligomeric structure that contains several TEMPO moieties.Its high molecular weight (between 2000 and 3000 g/mol) makes it particularly suitable for possible recycling without losing any oxidation efficiency. Generally, it is used in homogeneous conditions, and its high molecular weight allows easy recovery of the end products by simple distillation. The theoretical structure can be written as shown in Figure 2.

Catalyst Efficiency
The conditions that are generally followed for the use of Oxynitrox S100 correspond to a biphasic medium. The general procedure uses sodium hypochlorite as oxidant, Oxynitrox S100 as catalyst, dichloromethane, ethyl acetate or toluene as solvent, and sodium bromide as co-oxidant. This co-catalyst leads to the in situ formation of NaOBr, which is a more efficient oxidant than NaOCl. The degree of oxidation of the final product can be controlled by the amount of sodium hypochlorite: when using 1 to 1.3 NaOCl equivalents, or when using 2 NaOCl equivalents, the primary alcohol is respectively converted into the corresponding aldehyde or acid.The operating conditions are mild, and the reaction temperature can be adjusted from 0 °C to room temperature. The reaction requires the control of the pH (around 8 to 9.5) using a buffer solution.
The reaction model that is mainly presented in this article corresponds to the conversion of n-octanol into n-octanal (Figure 3). Various conditions were experimented on, and all results have been compiled into Table 1.
All these results demonstrate that only a small amount of Oxynitrox S100 (0.5% weight in comparison with the amount of n-octanol) is required to produce highly satisfactory results. A 1.1 sodium hypochlorite equivalent is enough to obtain selectively the aldehyde form; with the use of 2.5 equivalents of sodium hypochlorite, the acid is obtained as the main component of the reaction. As co-oxidant, NaBr is necessary to obtain a good yield. The optimal reaction temperature in this case is 20 °C, and the pH must be carefully controlled between 8 and 9. The best results are obtained by setting up the reaction in an appropriate solvent: data show that the use of dichloromethane can lead to a 93% yield in n-octanal after 1.5 hours.
With regard to recycling benefits, in this example, n-octanal can easily be recovered by distillation. As a consequence, the distillation bottom, which contains the catalyst Oxynitrox S100, can be recycled. Figure 4 shows the octanal yield obtained by repeating the oxidation reaction after two cycles.
In addition to aliphatic alcohols, other alcohols were also efficiently oxidized: the use of Oxynitrox S100 can be extended to benzylic alcohols, cycloaliphatic alcohols or unsaturated alcohols, and shows excellent yields and selectivities (Figure 5).

Applications
Oxynitrox S100, a high-performance catalyst for selective oxidation reactions, can lead to several applications in which the concepts of “sustainable development” or “responsible care” are paramount.5 Thanks to its countless advantages described above, it can be used in various nitroxide mediated oxidation fields such as pharmaceuticals,6 production of flavours and fragrances,7 cosmetics,8 agrochemicals, or, generally, polysaccharide oxidation.9
Conclusion
As the result of Arkema’s innovation, Oxynitrox S100 makes its own contribution to the demands of modern chemistry, which makes environmental protection a priority. Oxynitrox S100 is a versatile and innovative catalyst for oxidation reactions. High activities and selectivities are achieved for different types of alcohols and its use can be extended to polyols or carbohydrates. This catalyst is being developed by Arkema,10 and is currently produced at the pilot scale. Its many advantages make it very useful in organic synthesis and it is of particular interest in the field of specialty chemicals.This paper was published in Specialty Chemicals Magazine, Dec., 2006, 32-33.
For further information, contact Dr. Sandra Grimaldi, Corporate Business Development, Arkema, e-mail info.oxynitrox@arkema.com, or e-mail sandra.grimaldi@arkemagroup.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







