High-Solids Alkyd Strategy

This article presents new possible approaches for alkyd resin architecture in order to comply with market constraints related to VOC emissions. Examples and performance of alkyd concepts are presented with regard to VOC content and performance for “interior/exterior trim and cladding paints for wood and metal”.

Background
New regulations related to emissions/VOC content in coatings and increasing oil prices strongly recommend the shift to waterborne and high solids for coating manufacturing technology.In Directive 2004/42/CE, from the European Parliament and the Council, limitations are established for the maximum VOC in decorative paints to be used within the EU. The products covered by the Directive are for use on buildings, trims, fittings and structures associated with buildings. Specific sub-categories are listed in the Directive with different limitations for maximum VOC in g/L of the ready-to-use product with two sets of limits for each sub-category. The first set of limit values will apply from 1 January 2007 and the second, stricter set, from 1 January 2010. Limits are established for both solventborne and for waterborne paints. The target levels are presented in Table 1.
To reach these targets, along with the continuous development of waterborne systems is the possibility of finding new ways for high-solids technology.

Definitions
There is no general consensus for defining high-solids systems. However we list below some of the most commonly used.- A system is named “high solid” when it contains a max 350 grams VOC/litre of coating.10
- There are different regulations in the United States, e.g., max 420 g/L and max 250 g/L depending on application and state.10
- A paint is named “high solid” when it has a VOC content of max 420 g/L at application viscosity.9
- The phrase “high solid” sometimes is used for all kinds of paint systems with a non-volatile content higher than average. This is very misleading because the average non-volatile content differs from application to application.9
- A high-solids coating typically contains greater than 60% solids by weight or 80% solids by volume.8
- Lacquer with very high non-volatile content (>70%).11

Specific Approach for High Solids
Formulation of high-solids systems requires attention with respect to the choice of all involved components. Here we have listed some of the factors that influence the selection:- binders of high reactivity;
- binders of low viscosity/low molecular weight;
- molecule design;
- pigments/fillers of low oil index;
- wetting additives; and
- solvents that reduce the number of H-bonds.
Alkyds are type B polymers that exhibit high functionality but generally have a low glass transition temperature. The Tg and hardness are built up in the drying process.
In the oxidative process, the difficult part is that crosslinking does not
occur by reaction of the alkyd with another component (crosslinker) and by
subsequent removal of functional groups that have a plasticizing effect, but
through polymerization of double bonds (delivered by the fatty acids). Hence,
the final molecular weight is not as high as a thermoplastic polymer, and the
hardness development is not always as high as expected. The phenomenon occurs
as the air/oxygen kicks off the autoxidation of the alpha position from the
double bond on the fatty acids in a complex process of radical polymerization
assisted by metal dryers. This process results in the linkage between the fatty
acids whose bonds may be a carbon-carbon bond, an ether bond or a peroxide
bond. It is commonly accepted that driers only assist in the process of
hydroperoxide decomposition and that higher temperatures and highly conjugated
fatty acids favour the formation of carbon-carbon bonds.

Basically an alkyd binder has a drying diagram as shown in Figure 1, while a high-solids alkyd has a much longer drying time due to a very low Tg, which is built by the oxidative reaction as depicted in Figure 2.
Considering the drying process itself, and defining drying as an expression of viscous behaviour, according to the Williams-Landel-Ferry equation (Figure 3), the dry-to-touch performance at 25 ºC (viscosity measurement temperature) is achieved at a Tg as low as -29 ºC.

The viscosity of an alkyd resin is a function of free-volume availability. The free-volume of a material is the summation of the spaces or holes that exist between molecules of a material resulting from the impact of one molecule or molecular segment striking another. These holes open and close as the molecules vibrate. Above the glass-transition temperature (Tg) the holes are large enough and last long enough for molecules or molecular segments to move into them leading to low viscosity. Free volume increases as temperature increases; the rate of volume increase is higher above Tg or in case we use a solvent.

The answer may be the design of the alkyd binder itself. Basically this means moving from low free volume to higher/high free volume (Figure 4) in an almost 100% non-volatile content.
Allowing the alkyd binders to have a reasonable molecular weight and at the same time shielding a segment that has a reasonable high Tg, the hardness build up will be transmitted to the coating in the drying process. The shielding is necessary to provide the low viscosity, while the architecture itself must allow a sufficiently reasonable free volume, leading to a reasonable application viscosity in conditions complying to the high-solids definitions.


The pendulum is measuring oscillations in the soft shell of the molecule. Therefore the molecule should be shaped as much as possible, as in Figure 6, in order to allow the hard core of the molecule to be visible in the drying process.
Therefore, the alkyd binder should be built as a discoid structure (Figure 6), allowing a higher mobility and a lower shielding of the hard core of the molecule. The core may be shaped in order to control, by raw materials and length, the Tg. A shape as shown in Figure 7 may be very suitable to comply with these requirements and is easy to synthesize using the usual raw materials for alkyds, using semi-fabricates thereof, or loaded and reacted in a suitable order.
Considering the architecture shown in Figure 7, the molecular weight may be
grown on the dendritical side of the molecule, trying to preserve the
architecture as shown in Figure 8. This may be performed by reacting
hydroxyl-functional dentritical moieties of low OH number on
anhydride-functional moieties as will be further explained.



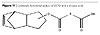

The further growth of molecular weight according to the architecture in Figure 8 is performed by reacting maleic anhydride on the fatty acid moieties in the dendritical structure as in Figure 10 and further reaction of the said dendritical polyol fatty acid esters to those moieties. The resulting moieties from the reaction of maleic anhydride on the fatty acids are presented in Figure 12.


Experimental and Discussion
In order to make a proper evaluation of the idea, a typical long oil alkyd was prepared and compared to alkyd binders obtained according to the concept. Furthermore, in order to maintain some functional reactive groups on the binders according to the concept, it was necessary to fulfil other requirements related to the coating formulation such as adhesion, pigment wetting, etc. For the 1,3-diol in the hard segment, the choice was to use BisMPA (dimethylol propionic acid) to control the synthesis in such a way as to preserve the larger part of the carboxylic group unreacted in direct or side reactions.
The concept alkyds may be synthesized in steps: the first step is to generate
the strong segment as described in Figure 9, which is further loaded to the
branched ester moieties of polyol and fatty acids. However it is possible to
run the synthesis in one shot by carefully observing the correct order of
loading the raw materials in order to lead to the desired structure at
temperatures close to, or slightly higher than, the melting point. Concept
alkyds have been synthesized according to the following protocol.


Drying Performance Evaluation of the Concept
Clear coatings were prepared as shown in Table 4; performance properties are shown in Figures 14 and 15.
The particles in the AFM images have a large size distribution. A likely
explanation is that the samples were too concentrated. Several polymer
molecules could then merge forming larger agglomerates. The large particles in
Figures 16 and 17 could be such agglomerates. However, when the sample was
further diluted, contact between the AFM tip and the sample could not be
achieved. Areas with few or no big particles could be identified, Figure 16,
and size analysis of these areas was performed and is shown in Figures 18-20.
The section analysis of the height images gives vertical sizes ranging from ca
1.8 µm to 4 µm for those small particles.


Conclusions
Considering the drying performance and the VOC content in formulations that are aromatic-free we may assume that the approach has been successful.
The drying performance of the typical alkyd
is lower than the performance of both concept alkyds as described above. The
blends of the typical alkyd with any of the concept alkyds may impart both
hardness and drying performance. The approach may comply with the request of
VOC emissions of 2007 and even 2010. Some trimming of the formulations may be
necessary and is left to the customer discretion, but this may be considered a
reasonable starting point. The binders as presented here are filling the drying
behaviour gap between typical alkyds and general high-solid alkyds or simple
dendritical structures. The concept binders may be used as such or in
combination with typical alkyds as reactive solvents without the risk of
altering the drying behaviour or other general properties.

For more information, visit www.perstorp.com.

Acknowledgements
The authors would like to thank the Market & Sales people (Per-Erik Velin, Kent Hamacek) from Perstorp Specialty Chemicals AB for encouraging this project, as well to Stefan Lundmark and Anna Andersson, from the University of Lund, Sweden.Bibliography
1. Hubert, J.C, Vanderbosch, RAM, Muizebelt, Klaasen, RP, Zabel, KH –J. of Coatings Tech., vol 69, no 869, June 1997.2. Hubert, J.C, Vanderbosch, RAM, Muizebelt, Klaasen, RP, Zabel, KH-Progress in Organic Coatings, 31, (1997), 331-346.
3. Krik Othmer Encyclopaedia - Dialog On Disk Book.
4. Patton- Alkyd resins, Interscience Publishers, 1962.
5. Air drying polymer- Perstorp Specialty Chemicals AB, patent EP0651039.
6. Tsuruk, Vladimir-Dendritic macromolecules at interfaces-Advances materials, 1998,10,3.
7. Rogunova,M, Lynch, T-Y.S, Pretzer, W, Kulzick,M, Hiltner,A, Baer, E,-J. of Applied Polymer Sci.Vol 77,1207-1217 (2000)M.
8. Bethencourt, F. J. Botana, M. J. Cano, R. M. Osuna & J. M. Sanchez-Amaya-Electrochemical evaluation of a high solid coating in acid media.
9. Brochure from Akzo Nobel, March 2003.
10. Alkyd/polyester surface coatings-CEH report 592.6000H.
11. Deutsche Lackinstitut (lacke-und-farben.de)
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





