When introduced, new coalescents are normally tested as a one for one replacement for the existing coalescent. Generally, no other formulation changes are made in a preliminary screen. This is a proper approach for a first pass. The new coalescent (or virtually any new raw material) must demonstrate at least acceptable performance under that constraint. However, new raw materials will often require "fine tuning" of the formulation for optimum performance to realize gains and improvements over existing art.
Velate 368, 2-ethylhexyl benzoate, as a new and improved latex paint coalescent alternative, was introduced to the coatings industry in late 1999.2-5 Like TMB, Velate 368 is not soluble in water, and has the following performance attributes.
As experience has been gained in working with Velate 368, formulation guidelines have been developed. This article presents information on formulating with Velate 368 in coatings. While the formulation steps are specifically aimed at incorporating the coalescent, the steps listed should always be considered in the development of any new formulation to gain a clearer understanding of coalescent needs.
Formulation Guidelines
1. Consider Coalescent Partitioning and Partition Rate Since TMB and other compatible water-insoluble coalescents must partition to the polymer phase of the coating to function, rate of partitioning to polymer is important.8-12 The process of partitioning to the polymer generally involves coalescent emulsification followed by adsorption and absorption. Surfactant(s) in the system can affect this process. Water-soluble types may also partially partition to the polymer but partitioning to the polymer is not necessary for these products to function nor is it necessarily desired.
When Velate 368 was first evaluated in acrylic and vinyl acrylic emulsions, no issues in partitioning and partition rate were noticed. However, when the Velate 368 was then evaluated in a harder styrene acrylic emulsion on a minimum film forming temperature (MFFT) plate, differences in partition rate were identified. The coalescent was simply stirred into the emulsion and drawn down on the plate immediately. The Velate 368 did not appear to function as a coalescent in this particular emulsion. This information seemed to be contradictory. The Velate was being sold commercially at this point in time and did function as a coalescent. As a result, a study was designed to determine why its performance was not what was expected in this one system.
The binary blends were prepared by simple mixing with a three-blade propeller stirrer at 500 RPM. Coalescent was added quickly to the base emulsion and a total of five minutes mix time was used.
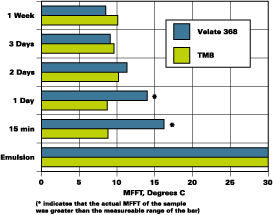
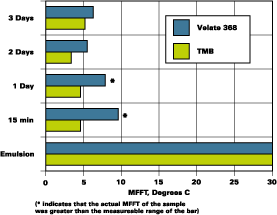
The partition rate of two emulsions was determined. One emulsion was a hard styrene acrylic emulsion with a MFFT of 30degC and a glass transition (Tg) of 40degC. The other is a softer vinyl acrylic with a MFFT of 12degC and a Tg of 19degC. Figures 1 and 2 illustrate the results of the MFFT vs. time for the styrene acrylic with two concentrations of coalescent. Figure 3 illustrates the results of testing one level of coalescent in the vinyl acrylic emulsion.
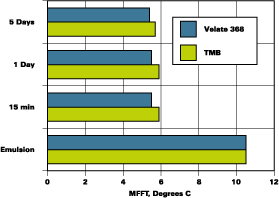
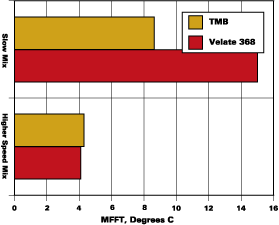
The blends used to develop the data shown in Figures 1-3 were prepared with minimum energy input during mixing. A coating made by normal preparation techniques with any of the resins noted above would receive more mixing energy input than was used to prepare the initial binary blends. An experiment to determine the effect of moderate mixing on coalescent performance was conducted with the systems that were slower to develop low MFFTs with 2-EHB. Two of these experiments are illustrated in Figure 4.
The MFFT data as presented in Figure 2 are included in Figure 4 as the slow mix data. The higher speed mix, 1,600 RPMs with a saw tooth blade for 30 minutes, is illustrated beside the slow mix data. It is clear that proper mixing ensured rapid partitioning of Velate 368 to this polymer.
This data as well as other data suggests that partition rate is not a concern in paint properly mixed, but some consideration to partition rate should be given.
2. Coalescent Efficiency
The determination of the proper amount of coalescent required for any coating application is important. If too little coalescent is used, improper film formation will result. Too much coalescent will yield higher VOCs and the possible degradation of performance properties. What is the proper approach to the determination of coalescent efficiency concentration? (Efficiency concentration is defined here as that level of coalescent required for adequate paint performance.) Any performance property affected by coalescent use could be an efficiency parameter. However, those tests relating to film formation are key.The affect of coalescent on the reduction of minimum film forming temperature on an emulsion has been used to determine relative efficiency of coalescents. However, measuring coalescent performance in paint is a more meaningful approach to evaluation of coalescent performance. The following tests are useful tests to measure coalescent requirements.
- Low temperature coalescence
- By visual assessment and/or
- By ASTM D 3793 porosity ratio
- Scrub resistance (ASTM D 2486, abrasive media with shim)
- After room temperature dry
- After low temperature dry
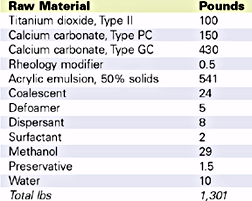
In this study, the low temperature porosity ratio as well as room temperature and low temperature dry scrub tests were used to determine the efficiency concentration of Velate 368. The ASTM porosity ratio test for low temperature is a particularly useful test to help quantify coalescence. Drawdowns dried at room temperature and low temperature are prepared and reflectance readings are measured. A portion of each draw down is stained and reflectance readings are taken over the stained area. The difference between stained and unstained readings on the low temperature chart is divided by the difference of readings on the room temperature chart is the porosity ratio. A value of one is best. Figure 5 is a picture of a draw down used for a porosity test.
The porosity ratio data indicates that Velate 368 is more efficient than TMB in both the flat and gloss paint. The low temperature scrub data indicates efficiency in the semigloss. The efficiency concentration of the Velate in the paints tested is as follows.
- Gloss - 50% of nominal TMB concentration.
- Semigloss - 25% less than efficiency concentration of TMB.
- Flat - 25% less than the efficiency concentration of TMB.
Reduction in coalescent level will affect VOCs of paint. The EPA 24 data on the flat paint is illustrated in Figure 6. Even at equal coalescent level, 2-EHB yields fewer VOCs from this paint. Coupled with the reduction in required level of coalescent a significant reduction of VOC results.
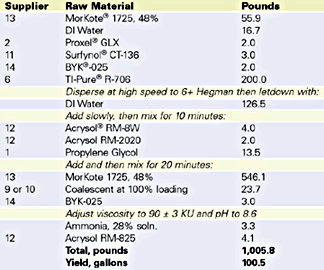
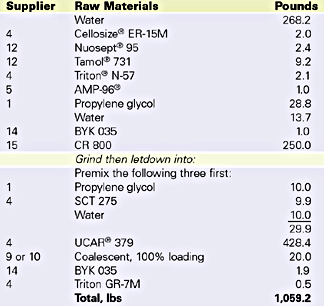
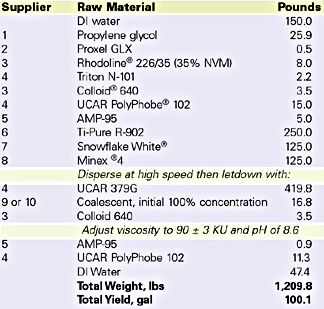
Velate 368 was also tested in traffic paint. An Illinois DOT formulation was prepared and evaluated for coalescent efficiency. The specification formulation is listed in Table 5. Low-temperature coalescence by visual assessment was used to determine the efficiency concentration of Velate 368. Since dry time is so important for a traffic paint, dry no pick up time (ASTM D 711), an efficiency test for traffic paint, was measured. The data is listed in Table 6.

Gains in efficiency will not be seen in all systems tested, but overall a reduction of coalescent use across a line of products is most likely. Determination of efficiency can be complicated by formulation factors such as formulations that have PVC's higher than CPVC. Other formulation variations can also affect system performance.

3. Determine if Glycol Reduction is Possible
Initial 2-EHB screening data implied that it might be possible to reduce glycol content in paints.13 Additional data confirmed that in some systems it is possible to reduce glycol content by 10-25% but this reduction is system dependent.14-15 Once the efficiency concentration for Velate 368 is determined in a formulation, a glycol ladder is suggested. Of course, glycol reduction will also reduce the VOCs in the paint, but the VOC reduction must be balanced with performance properties. Glycol is used in a paint for freeze/thaw resistance but also contributes to wet edge/open time. The reductions in glycol suggested above are significant, and may not lead to significant degradation of wet edge/open time. This, too, is system dependent.4. Consider Surfactant Demand of the System
In most instances, the current type of surfactants in a formulation is not an issue with Velate 368. However, since the coalescent is a different chemical type than TMB, some changes may be required to correct a surfactant imbalance, if one should occur. Such an imbalance may manifest itself as a change in color acceptance, color development or in other property development such as efficiency parameters. Color acceptance may actually be better with Velate 368, but formulators may want to adjust the acceptance to the level attained in the existing formulation. If a color acceptance or color development difference is evident we recommend testing different surfactant levels (above and below current level) of the existing nonionic surfactant with Velate 368 at the efficiency concentration.The HLB of Velate 368 is about 7; for TMB it is about 3. The required HLB for Velate 368 is 13.8, and for TMB it is 12.6. Selecting a surfactant with a lower or higher HLB than is currently being used may help to correct any surfactant imbalance. Since other factors exist that affect the surfactant balance, changing to a lower or higher HLB nonionic surfactant (2-3 HLB units) should adjust the difference, but testing is required to determine this.
5. Formulate Exterior Paint by the Above Steps
Exterior paints with Velate 368 can be formulated as with TMB and by the above guidelines. Exterior exposure of paint on a Florida fence began about two years ago, and no issues have been identified. No other differences in formulating to Velate 368 from TMB should be required.36. Run a Complete Evaluation on Adjusted Velate 368 Paints
It is likely that if the Velate 368 functions properly in the evaluation tests discussed, Velate 368 paints should function completely as well as, or better, than paints based on TMB. However, a complete evaluation as defined should be conducted.Conclusion
Velate 368 is a low-odor and lower volatility coalescent for latex paint. The material partitions to paint emulsion polymer as TMB does. However, the partition rate of Velate 368 is slower than TMB in some harder polymers but is just as quick as TMB in softer polymers. The partition rate of the Velate to polymer is accelerated by proper mixing, as would normally be associated with mixing paint during normal coating preparation. The paint efficiency data indicates that Velate is more efficient than TMB and the level of efficiency is formulation dependent. Since less coalescent is required and Velate 368 is less volatile, lower VOCs than paint based on TMB will result. It is also possible to reduce the level of glycol in some paints formulated with Velate 368, which can further reduce VOCs. To realize the gains in performance possible with Velate 368, it is suggested that paint formulators utilize the formulation guide and test protocol suggested to determine Velate 368 coalescent requirements.For more information on coalescents, contact Velsicol Chemical Corp., 2910 MacArthur Blvd., Northbrook, IL 60062; or Circle Number 143.
References
1 Eastman Chemical. "Texanol Ester-Alcohol Coalescing Aid for Latex Semigloss Paints," Publication No. M-132B2 Arendt, W.D. "New Low Odor Benzoate Coalescent for Latex Paint", Proceedings of the Twenty Seventh International Higher-Solids and Waterborne Coatings Symposium, March 1-3, 2000, New Orleans, p. 150-159.
3 Arendt, W.D. "New, Low Odor Coalescent for Latex Paint," XXV FATIPEC Congress Proceedings, Vol. 3, pp. 161-175, Turin, Italy, September 19-22, 2000.
4 Arendt, W.D. "2-Ethylhexyl Benzoate: New, Low Odor, Low Volatility Coalescent for Latex Paint", Proceedings of the 78th Annual Meeting of the FSCT, pp. 105-119, Chicago, October 18-20, 2000.
5 Arendt, W.D.; Strepka, A.M.; Gruszeecki (Riley), K. "Coalescent Formulation Studies: Efficiency and Partition Rates," Proceedings of the 28th International Higher-Solids and Waterborne Coatings Symposium, February 21-23, 2001, New Orleans, pp. 497-509.
6 Stanley, R. "R&D Helps Para Paints Be First to Market," PCI, Vol. XVII Number 6, pp. 68-70, June 2001.
7 Velsicol Chemical Corp., "Velate 368 Formulation Guidelines," July 2001.
8 Smith, L. "Predicting Cosolvent Efficiency for Coalescing Solvents," Proceedings of the 14th Higher-Solids and Waterborne Coatings Symposium, February 25-27, 1987, New Orleans, pp. 104-121.
9 Guthrie, D.H. et al. "Evaluations of Coalescing Agents for Industrial Latexes", Proceedings of the 14th Higher-Solids and Waterborne Coatings Symposium, February 25-27, 1987, New Orleans, pp. 77-103.
10 Hoy, K.L. "Estimating the Effectiveness of Latex Coalescing Aids," J. Paint Technol, Vol. 45, No. 579, April 1973, pp. 51-56.
11 De Fusco, A.J. "New Coalescing Solvents Advance Formulation of Latex Coatings," Modern Paint and Coatings, November 1989, pp. 56-66.
12 Kelyman, J.S. "Propylene Glycol Phenyl Ether As A Latex Coalescent," Modern Paint and Coatings, October 1986, pp.155-160.
13 Willner, J.H.; Sliva, T.J. " Evaluation of Velate 368 vs. Texanol in Vinyl Acrylic and Acrylic Latex Paints," August 27, 1999, Report DL-12335 (Available from author upon request).
14 Marschall, D. "Comparison of Velate 368 vs. Texanol in Two Interior Vinyl Acrylic Flats, and an Exterior Acrylic Semigloss," September 15, 2000 (Available from author upon request).
15 Marschall, D. "Glycol Reduction Project of Velate 368 vs. Texanol in an Interior Vinyl Acrylic Flat and an Interior Acrylic Semigloss" (Available from author upon request.)




