Nanomaterial Production and Properties
Nanophase Technologies produces nanocrystalline metal oxide powders by way of a patented Physical-Vapor Synthesis (PVS) process. The process involves vaporizing a metallic or metal-oxide composite precursor in a plasma, followed by rapid quenching to initiate condensation and formation of extremely small metal oxide crystallites. The size of the crystalline particles can be tightly controlled through variation in the condensation rate and the particle concentration in the downstream quench zone. The discrete metal oxide nanocrystalline particles form loose aggregates that allow them to be collected in a dry powder form. These loose aggregates can then be redispersed in solution to provide stable suspensions of the individual nanocrystalline particles. Because the PVS process uses metals or metal oxide composites as the only starting materials, any product contamination that may result from solvent or solvated precursors material is avoided. As a result, purity of the metal oxides nanoparticles, in both the bulk phase and on the surface, can be maintained at a very high level. In addition, the PVS process can be scaled up to provide a production rate on the order of tons/year. Examples of nanocrystalline oxides currently produced on a bulk scale include alumina, ceria, titania, zinc oxide, iron oxide, antimony/tin oxide, and indium/tin oxide. Numerous other pure oxides and mixed metal oxides are also accessible with the PVS process, and can be produced upon market demand.
Metal oxides prepared by the PVS process are comprised of crystalline, equiaxed, nonporous, discrete particles exhibiting mean diameters in the 10-50 nm range, with surface areas of 15-90 m2/g. The high surface area and small particle size provides a substantial surface/bulk atomic ratio, and results in a highly strained surface with numerous reactive sites.
Surface Treatment
Though nanocrystalline metal oxide powders have found use in a variety of coating applications, in most cases the powders require surface treatment prior to their incorporation into a product. Several proprietary surface treatment processes have been developed for metal oxide nanoparticles, designed to provide one or more of the following properties.- Dispersability in liquids (aqueous, alcohol and hydrocarbons)
- Prevention of particle agglomeration
- Compatibility with resin matrix
- Functionalization of oxide surface with reactive groups
- Refractive index matching
- Passivation of the oxide surface chemistry
The surface treatment process is also designed to enable compatibility of the particles with the resin film matrix. In certain cases, the surface treatment process is used to incorporate functional groups on the oxide particles, allowing for direct interaction with the resin polymers. For certain systems, refractive index matching is necessary to yield transparent coatings, and surface treatment chemistry can be used to minimize the particle/matrix refractive index difference. Finally, as mentioned earlier, the nanocrystalline oxide surface is very reactive, and in some coating systems this necessitates a surface treatment process to passivate this reactivity so as not to interfere with the film curing process.
Abrasion-Resistant Coatings
Conventional abrasion-resistant coatings often feature metal oxide particles incorporated within the resin film to suppress marring, scratching or abrading of the coating. Alumina is the preferred oxide for this purpose due to its extreme hardness (9 on the Mohs scale), and relatively low cost. The drawback to such alumina-containing films is that, although the abrasion resistance is often improved, the transparency of the coating is compromised due to the light scattering of the micron-sized alumina particles used. However, the use of nanometer-sized alumina in such coatings offers a solution to this problem since the particles are less than 100 nm in diameter, and thereby greatly reduces haze from light scattering. In addition, the alumina particles produced in the PVS process are spherical, which, combined with their small and uniform size, yields a more even film surface to further enhance the scratch resistance of the coating.The abrasion resistance properties of nanocrystalline alumina were evaluated by incorporating the particles in a crosslinked melamine-formaldehyde resin. Because melamine-formaldehyde resins are transparent and very hard, they are often used as protective coatings on non-flexible surfaces such as furniture and flooring. The alumina was first dispersed in water to disrupt the loose powder agglomerates, and then surface treated to attach functional groups to the oxide surface. The surface functionalization process was designed to prevent particle agglomeration during the film curing process. A TEM image of the coated alumina particles is shown in Figure 1. As the image shows, the spherical alumina particles are each coated with a thin uniform layer approximately 0.5-1 nm thick. The coated alumina particles were blended with the aqueous melamine-formaldehyde resin system at the desired concentration, from which films were drawn down on glass substrates at 1 mil thickness and cured by heating at 150¿C for 15 minutes. The cured film thickness was 9.6 mm.
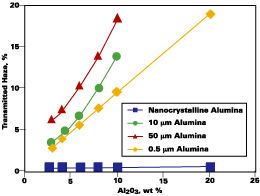
The transparency of the melamine-formaldehyde films containing nanocrystalline alumina was evaluated by measuring the degree of transmitted haze. The effect of alumina loading on percent haze in melamine-formaldehyde film is shown in Figure 2. As is evident, incorporation of up to 10 wt% nanocrystalline alumina particles has very little impact on haze (< 0.5%). In fact, alumina loadings up to 30 wt% have been achieved without increasing the film haze by more than 1.0%. By contrast, more conventional alumina particles in the micron range were found to impart significant haze to the film. In addition to the transparency afforded by nanocrystalline alumina, the film hardness was also found to increase substantially. Using the pencil scratch test, melamine-formaldehyde films containing 20 wt% nanocrystalline alumina were shown to be 4-6 units more scratch resistant than films without the nanocrystalline alumina.
The combination of high crystallinity and extremely small particle size of alumina produced by the PVS process positions this material to be uniquely suited to coatings applications where high transparency and high abrasion resistance is required. Nanocrystalline alumina has been formulated into several commercial abrasion-resistant coating products with numerous other potential applications currently under development.
Editor's Note:This article was originally published in Business Communications Co.'s Nanoparticle News (July, 2001, vol. 4, no. 6), www.bccresearch.com.
For more information on nanomaterials, contact Roger H. Cayton, Nanophase Technologies, 1319 Marquette Drive, Romeoville, IL 60446; phone 630/771.6707; visit www.nanophase.com; or Circle Number 142.




