Design of Wetting and Dispersing Additives for Selected Pigments

- Efficient wetting of pigment surfaces during incorporation into aqueous formulations,
- Efficient development of maximum color strength, and
- Long-term shelf stability of the pigment concentrates.
- High-gloss and low-haze levels determined by the compatibility of the wetting and dispersing additives with the resin systems and pigment particles,
- Weatherability: UV- and water resistance, and
- Compatibility for use with a variety of color shades.
- They must migrate to the pigment surface to ensure coating integrity.
- They must not be replaced by other polymeric substances, such as resins.
- They must not be solubilized with the addition of co-solvent.
- They must not break down pigment stability during the coating film drying process as the material changes from water-thinnable to water-resistant.
Design Synthesis of Wetting
and Dispersing Additives
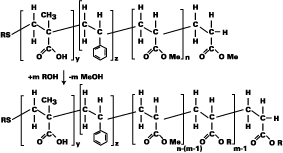
Several routes for the synthesis of polymeric wetting and dispersing additives exist. Acrylic copolymers can be engineered using different hydrophobic (e.g., styrene/butyl acrylate) and hydrophilic monomers (e.g., carboxylic acid groups after neutralization). After polymerization and neutralization of the acid groups this approach yields aqueous products. The non-polar portion of these molecules adsorbs to the polar parts, while the polar portion dissolves in the aqueous media, creating a stabilizing shell around the pigment particle. If the substances contain acid groups, they stabilize pigment by repulsion of the charged surfaces.
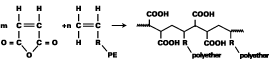


Such products are free of ester linkages, which are unstable against hydrolysis. They can be produced in a few steps from raw material sources at attractive price levels. This synthetic route requires no VOCs.
Changing the vinyl polyether design (e.g., using hydrophobic alkylene oxide monomers) can make the polyethers compatible with different kinds of pigment surfaces.
Model structures from these different synthetic groups were compared in application tests and analytical measurements made for their pigment stabilization performance (see Table 1).

Methods for the Determination of Adsorption Properties
Particle charge detectors from Mutek can measure the streaming potential of highly concentrated formulations (thus, close to real-life conditions). The equipment determines differences in the mobility of counter ions on a charged surface (leading to the formation of dipoles under high shear forces).2-4 In comparison to other methods described in the literature, this method permits adsorption measurements without requiring the strenuous separation of pigment concentrate particles from the surrounding liquid phase.5-8
Titration of aqueous pigment suspensions with additive solutions can determine differences in the adsorption behavior. After adsorption of the wetting and dispersing additives, addition of polyelectrolytes (until electro-neutrality is achieved) can quantify charge intensity on the pigment surface. Addition of substances, such as the surfactants found in resins, provide results about the additive's resistance to replacement from the particle surface.

Microcalorimetric experiments, which detect energetic effects of the additive adsorption on the pigment surface, are performed in mixing cells and provide information about the strength of interaction between additive and particle surface. These methods are common practice in biochemical studies.9-10
Test of Application Properties
The formulation of pigment concentrates using transparent iron oxides, transparent titanium dioxides, and other pigments with small particle sizes and high surface areas remains a challenge. To optimize transparency and brilliancy, the conventional grinding process makes high pigment loadings difficult to achieve. In addition, after storage, these formulations often exhibit dramatic viscosity increases.
As a first step, the additives were tested for performance in a formulation with one standard pigment type and high pigment content (see Table 2).

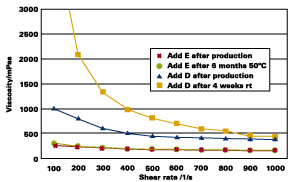
The high transparency achieved with additive E is easily seen in comparison to a commercially available product (see Figure 6).
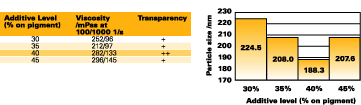
Similar results were obtained with other pigments from other suppliers. Among these, the rheological profiles of yellow iron oxides are, in general, more difficult to optimize, leading to slightly lower levels of pigment loading in the formulations (30-40%). The additive level must be optimized for each formulation to reach the maximum level of transparency as shown for Sicotrans L 1916 (see Figure 8).
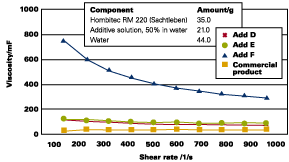
Transparent titanium dioxides used to achieve a so-called frost effect in metallic basecoats are likewise not easy to stabilize. Formulations with additive E and D show excellent transparency, as well as excellent rheological profiles (see Figure 10). Pigment particle size in the formulations is smaller than in commercially available concentrates (see Figure 11).

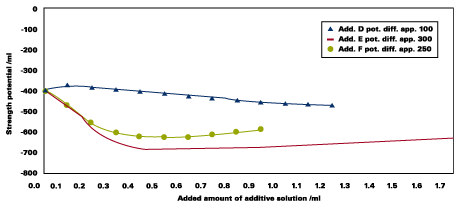
Pigment Slurries - Adsorption Properties
When additive solutions are added to aqueous pigment slurries, the copolymers E and F - containing vinyl polyethers - show strong shifts in streaming potential measurements. In comparison to additive D, adsorption on the pigment surface is much stronger for additives E and F (see Figure 12).
Conclusion
The copolymerization of vinyl polyethers with maleic acid anhydride in an aqueous solution is a new concept for the synthesis of customized wetting and dispersing additives. This concept provides structures that are hydrolytically stable and economically attractive.They show superior performance compared to other types of dispersing additives, e.g., for the formulation of pigment concentrates with transparent iron oxides. They also provide the desired rheological profiles at high pigment loadings together with excellent transparency. These honeycomb-like, block copolymers do not show significant foam stabilization like many other surfactants. Therefore, no defoamers are required in the formulations. Research opportunities to change the design of the vinyl polyethers offer the possibility to match the requirements of different pigment surfaces.
For more information on pigment additives, contact Tego Coating & Ink Additives, 914 East Randolph Street, Hopewell, VA 23860; phone 800/446.1809; fax 804/541.2783; e-mail frances. eggleston@us.goldschmidt.com; visit www.additiweb.com; or Circle Number 133.
References
1 Internal Report, Goldschmidt AG, EP0736553 A2.2 M?tek: Messung von Partikelladungen, Keramische Zeitschrift 5/1995.
3 James, M.; Hunter, R.J.; O'Brien, R.W. Languor 8 (1992) P. 42.
4 Osterhold, M.; Schimmelpfennig, K. Farbe und Lack, Volume 11 (1992) P. 841.
5 Osterhold, M. Farbe und Lack, Volume 8 (1995) P. 683.
6 Reck, J.M.; Dulog, L. Farbe und Lack Volume 2 (1993) P. 95.
7 Binford, J.S.; Gessler, A.M. J. Colloid Sci. Volume 63 (1995) P. 1376.
8 van den Haak, H.J.W. J. Coatings Technol., Volume 69 (1997) P. 137.
9 Mohsen, N.M.; Craig, R.G.; Filisko, F.E. J. Biomedical Materials Research, Volume 40 (1998) pp. 224-232.
10 Tewary, Y.B. et al, J. Physical Chemistry, Volume 99, Issue 5 (1995) pp. 1594-1601.
11 Internal Report, Goldschmidt AG, EP0736553 A2
1 M?tek: Messung von Partikelladungen, Keramische Zeitschrift 5/1995
12 M. James, R.J. Hunter, R.W. O'Brien, Languor 8 (1992) P. 42
13 M. Osterhold, K. Schimmelpfennig, Farbe und Lack, Volume 11 (1992) P. 841.
14 M. Osterhold, Farbe und Lack, Volume 8 (1995) P. 683.
15 J.M. Reck, L. Dulog, Farbe und Lack, Volume 2 (1993) P. 95.
16 J.S. Binford, A.M. Gessler, J. Colloid Science Volume 63 (1995) P. 1376.
17 H.J.W. van den Haak, J. Coatings Technol., Volume 69 (1997) P. 137.
18 N.M. Mohsen, R.G. Craig, F.E. Filisko, J. Biomedical Materials Research, Volume 40 (1998) pp. 224-232
19 Y.B. Tewary et al, J. Physical Chemistry, Volume 99, Issue 5 (1995) pp. 1594-1601.
Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






