Polymer Binder Key to Erosion Behavior
The film-forming polymer product is the key ingredient in determining the seawater erosion behavior of self-polishing marine antifouling paints. Self-polishing behavior of the paints is achieved through the erosion process of the polymeric binder, enabling the release of incorporated biocides at a constant rate. These paints prevent the growth of marine life and subsequently minimize corrosion on the hulls of seagoing vessels. Preventing biomass accumulation allows for:- A reduction in the effective drag on the vessel (improving fuel efficiency);
- A prohibition of foreign species from being transferred to non-native waters (e.g., prevention of zebra mussels entering the Great Lakes);
- An improvement in vessel maneuverability; and
- A predictable delivery time for ship owners.
Polymers that incorporate tributyltin groups, or TBT, into the pendant groups of their backbones were effective for two reasons:
Taken together, these characteristics led to a thin paint film "leached layer" (in the range of less than 20 microns) that delivered a controlled, minimal amount of biocide at a constant rate. The leached layer is established when the biocide front moves at the same rate as the polymer front.
Environmental Concerns With Existing Technology
Environmental concerns regarding the release of TBT byproducts into seawater led the Marine Environmental Protection Committee (MEPC, connected to the United Nations) to propose that the application of marine paints containing TBT-based products be prohibited by 2003 and the presence of the paint on ships to be banned by 2008. While the necessary number of countries has not ratified this treaty, several countries (e.g., the United States and the European Union) have voluntarily implemented the ban. Notably, all major paint manufacturers embraced the ban by no longer selling any tri¬butyltin-based paints and have marketed their own non-tributyltin paints that cost at least twice as much without the same long-term performance. This resulted in the market withdrawal of tributyltin-based copolymers in virtually one year.
Since the proposed MEPC tributyltin ban, the marine antifoulant market has segmented differently due to the costs and needs of the specific ship application. While existing commercial self-polishing marine antifoulant paints incorporate tributyltin-free binders and are sold into each segment, all of these paints (and, therefore, the binders) are performance deficient, too expensive, or both. Arkema's silyl products aim to provide tributyltin-like performance at a lower cost without the environmental consequences of tributyltin polymers.4

Application to a Ship Hull
The Arkema silyl acrylate polymers, designed as the key binder components for self-polishing marine antifoulant paints, were first available for orders and ship-hull applications in May 2004. The photographs in Figure 1 depict the ship patch application of paints containing two of Arkema's silyl acrylate products when first applied and after nine months. Additional data indicates that after one year of exposure the experimental patches are not fouled and color retention is superior to the commercial paint on the ship. The outstanding ship-hull performance is due to the pigment/biocide/polymer characteristics. In similarity to the tributyltin systems, the physical and chemical properties of each of the silyl acrylate polymers are designed such that the resulting paint film slowly and consistently erodes over time when immersed in seawater. In contrast to the tributyltin polymers, the controlled erosion rate of 1-5 microns per month allows the paint to deliver a constant, critical amount of incorporated biocide without the release of TBTO. The amount of released biocide is the minimum required to prevent ship-hull biofouling, while avoiding detrimental environmental effects caused by unnecessary biocidal over-release.

High-Throughput Research Contradicts Accepted Mechanism
The determining features for controlling erosion rate are a complex combination of many factors including polymer hydrophilicity/hydrophobicity, silyl group hydrolysis rate, dissolution (under shear) of the hydrophilic polymers generated, polymer molecular weight, polymer-chain entanglement, and phase behavior under water, etc.In the past, the mechanism of erosion for acrylate-based self-polishing binders containing hydrolysable monomers was proposed (Figure 2). As seawater slowly attacks the hydrophobic surface of the coating, the water-reactive groups are hydrolyzed and form free pendant carboxylates in the polymer backbone. The resulting hydrolyzed-polymer surface layer becomes very hydrophilic and swells with water. Once swelled, this layer shears off to reveal a "new" hydrophobic, non-reacted film surface. The layer-by-layer shearing action leads to a slow, constant release of the minimal amount of suspended biocide required for efficacy. Two natural conclusions of this proposed mechanism are as follows:
- In order for effective erosion to occur, a minimal level of 25 mol % hydrolyzable pendant groups (e.g., tri¬butyltin carboxylate groups) must be incorporated into the polymer.
- Once past this minimum level, increasing or decreasing the level of hydrolyzable groups should speed up or slow down the rate of erosion.
To explain these observations, Arkema used modeling calculations to show that the critical water-solubility limit of the partially hydrolyzed polymer can be manipulated through hydrophobic/hydrophilic selection of the water-reactive silyl group. In other words, the laboratory determined that the erosion rate could be selected not only based on the contribution that the silyl group makes to the polymer hydrolyzability, but also based on the contribution it makes to the water-solubility properties of the partially hydrolyzed polymer.
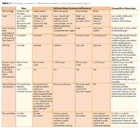
Comparison With Existing Technologies
The Arkema technology compares favorably to the two dominant technologies in the self-polishing market, copper acrylates and high-silyl-content silyl acrylates (Table 1).From a performance perspective, binders with a high content of silyl acrylate solve many of the performance deficiencies that the less expensive copper acrylate systems exhibit. Specifically, the hydrolysis-controlled, thin erosion zone is virtually identical to the characteristics of superior, environmentally controversial tributyltin polymer films without the concerns of organotin sediment accumulation. As a result, the antifouling performance is considered the best in the industry. Unfortunately, the production cost of the existing market silyl acrylates is exorbitantly high. This is a direct result of using an expensive silyl acrylate at levels of 40-50 mol % in the final product composition. Interestingly, despite the high amount of hydrolyzable monomer in the polymer, most silyl paints on the market still require rosin to allow for proper erosion. The addition of rosin degrades the controlled erodability of the final paint system.
The Arkema silyl acrylate products combine the best characteristics of both existing copper and silyl systems currently on the market. The Arkema products incorporate three to five times less of a specially selected silyl acrylate monomer than that of the existing silyl systems; this leads to a significant decrease in binder cost for the Arkema product. The product also does not require the use of an erosion rate-enhancing additive (rosin); hence, the erosion behavior of the paint coating is controlled solely by the chemical and physical properties of the polymer. Thus, Arkema's silyl acrylate compositions solve the cost and minor performance problems of the existing silyl systems, while meeting the economic constraints set by the less expensive copper systems.

The performance difference between a non-optimized paint formulation made with the Arkema binder technology vs. existing commercial paints in static testing was conducted in south Florida (Table 3). The data clearly shows that the experimental Arkema paint formulation exhibits comparable or better static-immersion performance than the commercial controls.

References
- Arkema team that contributed to the R&D 100 Award: Mike Abrams, Mark Aubart, Jean-Michel Chabagno, Marisa Hull, David Mountz, Ron Siebenlist, Gary S. Silverman, Dana Swan, Scot Swan, Ken Tseng, and Mei Wen.
- "Antifouling Technology - Past, Present, and Future Steps Towards Efficient and Environmentally Friendly Antifouling Coatings"; Yebra, D. M.; Kiil, S.; Dam-Johansen, K. Prog. Org. Coat. 2004, 50, 75 and references therein.
- "The Squeeze of Tributyltins"; Rouhi, A. M. Chem. & Eng. News 1998, 17, 41.
- (a) Aubart, M. A.; Guittard, E. P.; Silverman, G. S.; Tseng, K. K. US Patent 6,767,978, 2004. (b) Aubart, M. A.; Abrams, M. B.; Silverman, G. S., Obiols, J.; Tseng, K. K.; Mountz, D. A. US Patent Appl. 2004/0138332, 2004. (c) Aubart, M. A.; Abrams, M. B.; Silverman, G. S.; Obiols, J.; Tseng, K. K. US Patent Appl. 2003/0225184 (d) Silverman, G. S.; Abrams, M. B. US Patent Appl. 2003/0109597, 2003. A special thanks to Mei Wen and Arkema's analytical group for the SEM/EDX work.




