Adding Value to Industrial Coatings by Using Epoxy Functional Silicone Resins

Coatings used on petrochemical manufacturing facilities, oil-drilling platforms and above-water marine applications generally face exposure to corrosive environments from water and/or harsh chemicals, as well as intense radiation exposure from sunlight. Standard coatings last approximately 7 years for topside (above-water applications), and 3 years for applications that have continuous exposure to water (splash zone). Coating failure can be attributed to either cracking due to temperature variations and exposure to water, abrasion, pinholes during drying, or delamination of the film due to over application or high film build.

Coatings designed for the marine market have typically been formulated with an aromatic epoxy blended with mono or difunctional reactive diluents, silicone alkyds, aliphatic urethanes, and condensation-curable polysiloxane systems. Coatings utilizing the latter two chemistries have increased UV resistance and chemical resistance, however, there is still a problem with cracking in thicker films, and in some cases they do not meet the ever-changing VOC regulations. However, the use of silicone resins with epoxy functionality in conjunction with acid-functional acrylics increase resistance to cracking and improve UV resistance without sacrificing VOC.
The new silicone compounds are addition-curing materials, which do not require a moisture curing process. The replacement of the Si-OC bond with a Si-CCC bond increases the coatings ability to withstand attack from harsh chemicals such as sulfuric, phosphoric, and hydrochloric acids and ammonium hydroxide. In addition to chemical resistance, the more hydrolysis-resistant version increases the UV resistance of the coating. This is a requirement for protection of substrates in industrial and marine applications.

Background
The types of coatings currently used to protect metal substrates in the maintenance marine market are based on either one of, or a combination of, zinc-rich primers, epoxy primer/topcoats, silicone alkyds, urethanes and condensation-curable polysiloxanes.Generally, coatings developed primarily for corrosion resistance utilize both a zinc-rich primer and an epoxy topcoat. In cases where extremely high chemical resistance is required, an epoxy/novolac topcoat can be used. The inorganic or organic zinc primer controls corrosion through cathodic protection. The epoxy topcoat acts as a sealer for the zinc primer to protect the substrate from exposure to the elements associated with corrosion. However, these coatings often fail when exposed to sunlight.
Urethanes have been used as topcoats with epoxy/zinc coatings when the application requires corrosion resistance and resistance to UV light. These materials are considered to be toxic due to the possibility of minute amounts of free isocyanate present, and they are generally high in VOC. The Norwegian government's NORSOK M-501 initiative has indicated that they are moving away from this technology and focusing on polysiloxane systems for topcoats.
Coatings based on silicone alkyds have been used for applications requiring an ambient cure schedule in addition to high temperature resistance. The silicone alkyds are often used when both the UV light resistance, and high temperature resistance are required. However, in the presence of water and heat, the silicone-alkyd polymer may break down into its starting components. Once this occurs, the alkyd will continue to oxidize and form water-soluble polymers.

The newer technology utilizes a silicone resin (polysiloxane) with epoxy functionality as the binder, which relies on an addition-curing mechanism for crosslinking. Utilizing such a mechanism as the primary form of curing eliminates the concern for cracking in high-film-build applications. Using the epoxy-functional silicone resin as the crosslinker in acrylic-based coatings provides improved corrosion resistance, UV resistance and low VOC. This type of technology is also in line with the NORSOK M-501 initiative being a non-isocyanate system.
Silicone Terminology
The term "silicone" refers to compounds that have the empirical formula R2SiO, which is analogous to organic compounds with the formula R2CO, or ketones. Silicon is similar to carbon in that it is tetravalent; however in its ground state there is no evidence that supports the formation of a double bond. Therefore, the true empirical formula should be written as R2SiO2(1/2) with four single bonds to silicon.
Silane is a term given to compounds composed of a single silicon atom, and, depending on the number of Si-O bonds, can be further sub-classified as M, D, T or Q units (Figure 1). The Si-O bond is considered to be the functional site and, therefore, silanes with a single Si-O bond are referred to as monofunctional or "M" units. Silanes that contain two Si-O bonds are referred to as difunctional or "D" units, and the trifunctional silanes are referred to as "T" units. The tetrafunctional or quadrifunctional silanes are referred to as "Q" units. Another term given to silanes that contain a silicon-oxygen (siloxy-) bond is siloxane. Two silicon atoms bonded to each other through an oxygen bond is referred to as a disiloxane, and three siloxanes is a trisiloxane. Greater than three siloxane units are normally called a polysiloxane.

Silicone Polymers
Types of Polymers Fluids, elastomers and resins are the three main types of polymers produced and sold by silicone manufacturers. Silicone fluids are linear compounds, which are composed of mainly D units with the ends reacted with M units (Figure 2) to render the material non-reactive. Reactive silicone fluids are fluids that have not been end-capped and, therefore, are silanol functional. In some cases they have been end-capped with an M unit containing organic functionality. The reactive fluids are the building blocks of elastomeric materials such as sealants, caulks and elastomeric coatings. One of the unique attributes of polydimethylsiloxane is the extremely low surface tension, which can be attributed to its low rotational energy requirement. This enables the polysiloxane to orient itself at the liquid-air or solid-liquid interface to eliminate surface tension gradients developed during application and drying of the film. This makes linear silicone polymers excellent candidates for paint additives.
Room temperature vulcanized (RTV) or elastomeric compounds are linear polymers that have been end-capped with T units. The T units can crosslink to form elastomeric materials such as sealants.
Silicone resins are composed of predominantly T units and can contain some D or Q units. The increased concentration of T units increases the number of high-energy silicon-oxygen bonds, and, therefore, increases the polymer's resistance to UV and thermal degradation. The Si-OSi bond strength is 108-110 Kcal/mole, whereas the C-CC bond is 82-85 Kcal/mole, and the C-OC bond strength is 84-87 Kcal/mole.
Organic Substituents and High Temperature Resistance
The type of organic substituent bonded to the silane will determine whether it will be used to make a water repellent, coupling agent or polymerized to form a binder for high-temperature-resistant coatings. Silanes with methyl substituents are typically used to make methyl resins, and are often used when stain resistance or anti-graffiti properties are needed. The disadvantage of using methyl resins is that they are very incompatible with organic resins, and tend to form fisheyes when used as a blending resin. On the other hand, the incorporation of phenyl groups increases the polysiloxanes' compatibility with organic polymers by increasing the organic content. The phenyl substituent will also increase the polymer resistance to thermal degradation (Table 1). The half-life of phenyl substituents is 100,000 hours (11.41 years) when exposed to 482 °F (250 °C), whereas the methyl substituents have a half-life of 10,000 hours (1.14 years).
The incorporation of phenyl substituents on the silicon atom increases high temperature resistance and chemical resistance of the polymer. In addition, the phenyl substituent increases the compatibility of the silicone with organic polymers such as acrylics, fluorocarbons and polyesters. The use of di-functional dimethyl groups increases the flexibility of the coating, which increases the resistance of the polymer to cracking. The use of monomers containing reactive organic substituents provides the chemist the option of changing the curing mechanism of the polymer.

Film Formation
Silicone polymers cure to form coatings or silicone-modified organic resins by one of, or a combination of, the following reactions: hydrolysis and homopolymerization, hydrolysis and copolymerization, and addition reactions.The alkoxy-functional group on silanes or silicone polymers will react with water under ambient conditions to form silanol groups and generate alcohol as a byproduct (Figure 3). The silanol functional siloxanes will homopolymerize to form polysiloxane or high-molecular-weight silicone resin. Generally, the atmosphere provides moisture and, therefore, in thick films there can be a problem with through-cure. The loss of alcohol is responsible for film shrinkage, resulting in cracking of the coating. The modification of this type of system with organic polymers decreases the tendency for this to occur, however, it is not eliminated.
If the silicone is silanol functional then the byproduct of the reaction will be water. Generally, the silanol functional polymers are either a flake or in a high-pH or low-pH environment. These resins are excellent for use as blending resins or modifiers.
In each case the alkoxy or silanol functional siloxane will react with hydroxyl functional organic polymers to form silicone-modified resins (i.e. silicone-modified alkyd or silicone-modified polyester), as seen in Figure 4. This reaction is driven by heat and in both cases is a reversible reaction. The silicone-oxygen-carbon linkage is susceptible to hydrolysis, and the addition of moisture and heat can hydrolyze the material to form the reactants.
The incorporation of an addition-curing group on the silicone polymer will eliminate the reversible reaction and still allow the chemist the ability to form an ambient curing system. The formation of an epoxy functional silicone resin, where the epoxy group is attached to the silicone through the hydrolytically stable Si-C linkage and not the hydrolysis susceptible Si-OC linkage, will react with standard epoxy hardeners (Figure 5). Systems prepared using the epoxy silicone/hardener addition-curing mechanism have increased chemical resistance, corrosion resistance and UV resistance when compared to standard urethane and epoxy systems.

Discussion
The modification of silicone polymers with organic functional groups provides the coatings chemist with an alternative route for crosslinking siloxane polymers. The replacement of the low-energy C-C-C linkage with the high-energy Si-O-Si linkage through a stable bonding mechanism increases the coatings corrosion resistance and UV resistance. The flexibility of the polymer can be modified by the siloxane backbone or changing the type of co-binder (crosslinker).The ratio of tri-functional (T-units) silicon to di-functional (D-units) silicon is balanced to maximize the amount of high-energy Si-O-Si concentration without sacrificing flexibility. The high level of T units increases the polymer's ability to form a durable, chemical resistant coating. The incorporation of D units increases the polymer flexibility (Figure 6).
The use of alkoxy-functional siloxanes as the primary binder will result in cracking of the coating when applied in thick films. The replacement of the alkoxy-functional crosslinkers with addition-curing mechanisms eliminates the tendency for the coatings to crack and increases the chemical resistance and UV resistance of the polymer.
The use of alkoxy-functional silanes blended with polysiloxanes will have a tendency to crosslink during exposure to UV light and will result in cracking and delamination when applied in thick films. The use of organofunctional silanes as a crosslinker will also have a tendency to crack in thick films during exposure in QUV. In each case, the coating studied was high gloss and did not appear to have suffered degradation due to UV light but rather hydrolysis due to heat and water. Therefore, boiling water was used as a test to screen possible siloxane chemistries for resistance to hydrolysis.

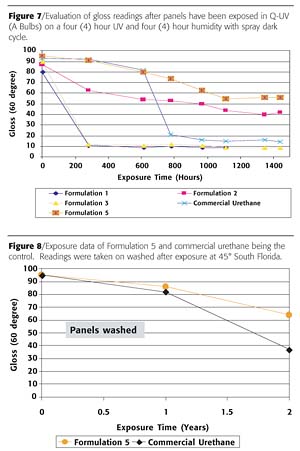
The use of epoxy-functional silicone resins as the hardener for organic systems such as acid-functional acrylics improves UV resistance, chemical resistance and adhesion. The acid to epoxy ratio should be kept at 0.67-0.74:1 in solvents. However if a reactive diluent is used, such as a polysiloxane, then the epoxy to acid group equivalents ratio should be increased to 0.85:1 (Formulation 5).

The exposure of these systems to QUV and South Florida indicated that the best-performing product was the epoxy-functional silicone resin with acid-functional acrylic. The commercial epoxy obviously failed QUV resistance, and the glycol-modified amines formed a hazy appearance early due to water uptake. The urethane looked good for a period of time, however it failed before the epoxy functional silicone resin/acid functional acrylic (Figure 7).

The color difference, or delta E (DE), measurements were taken on the washed and unwashed portion of the panels. The urethane had a significant increase in DE due to dirt retention and oxidation (Figures 10 and 11). The DE improves (decreases) on the epoxysilicone/acrylic (Formulation 5) due to the ease of cleaning and less dirt retention and less oxidation.

Applications
Epoxy-functional silicone materials are being used in maintenance marine and industrial maintenance applications. Companies, such as NAVCOR, Inc., located in Clearwater, Fl., have developed a product using epoxysiloxane technology called "CorroGuard EP609." They are using this product as the binder system in coatings for off-shore oil-drilling platforms. They have found the new technology "exhibited better adhesion to dry, and especially wet substrates, than the polyurethane technology in addition to it being isocyanate-free. "This finding opens up many new application possibilities not currently available with traditional systems," per Leo Meilus, the author of the paper entitled "Innovative Solutions for Anticorrosive Protection Using New Generation Coatings." His presentation at the Corrosion/2004 NACE show in New Orleans focused on the improvements made with the new epoxy siloxane technologies. The yellow railing and piping on the floating platform in Figure 12 was coated in 2003 and still looks new. The ability of companies to utilize siloxane in urea technologies has made it possible to develop products that have unique characteristics.

Conclusion
The protection of metal substrates is a growing concern for the industrial markets. The U.S. Navy alone spends $250 MM/year on corrosion protection and is desperately looking for ways to minimize these costs. There are also several cost reduction initiatives throughout the industry, such as the Norwegian government's NORSOK M-501, and the British Cost Reduction in the New Era (CRINE) project. The improvement of the conventional urethane, urea and/or epoxy systems used in these applications can be accomplished by replacing part of the organic binder with an epoxy functional silicone resin, thereby reducing the applied cost.Credits
Special thanks to NAVCOR, Incorporated and Engineered Polymers International (an Amber Chemical Group Company) for supporting information and photographs.
Special thanks to Rohm and Haas Company for their technical support and for supplying samples.
References
1 Kusumgar, M.; Nerlfli, S.; Downey, M. "The US Paint and Coatings Industry Multiclient Study, 2001-2006", November, 2002.2 Chang, B.; Guy, C. "Evaluating Maintenance Coatings for Offshore Platforms in Gulf of Mexico," J. of Protective Coatings and Linings, Volume 17, Number 2, February 2000.
3 Webb, A.; Brinkerhoff, B. "Reducing Navy Fleet Maintenance Costs with High-Solids/Solvent -Free Coatings Using Plural-Component Spray Equipment," SSPC Conference, 2002.
4 Doble, O.; Kvaerner, A."Coating Selection in the Norwegian Offshore Industry: Where, What, and Why", J. of Protective Coatings and Linings, April 2004, p. 22.
5 Funke, W.E. J. Coatings Technol. 1988, 60, 69-76.
6 Koerner, G. Silicone - Chemie und Technologie, Vulkan-Verlag, Essen, 1989, Ullmann, Paints and Coatings, Vol. 18, 438ff.
7 EP 291941, 1988, M. Wolfgruber, B. Deubzer, V. Frey (Wacker Chemie GmbH).
8 DE 4040986, 1992, K. Mautner, B. Deubzer (Wacker Chemie GmbH).
9 DE 4338421, 1993, M. Geck, J. Dauth, B. Deubzer, H. Oswaldbauer, M. Schmidt, F. Baumann (Wacker Chemie GmbH).
10 Geck, M.; Deubzer, B.; Weis, J.; Angew, Makromolekulare Chemie 1994, 223, 203-216.
11 Geck, M.; Deubzer, B.; Weis, J.; Pepperl, G. Organosilicon Chemistry II, VCH Weinheim, 1996, 673 - 684.
12 Laubender, T.; Göblmeier, W. "Use of polysiloxanes in powder coatings," Publication in PPCJ Paint & Ink International, May/June 1996.
13 Lawrence, R.E. "Meeting the Challenges of the 90's with VOC Compliant Silicones," American Paint and Coatings J., July 8, 1991, p. 36.
14 Hare, C.H. "Silicone Resins," J. of Protective Coatings & Linings, January 1995, p. 79.
15 Duvic, N.C. "Polysiloxanes for Coatings," European Coatings J., October 1995, p. 703.
16 Finzel, W.A. "Properties of High Temperature Resistant Silicone Coatings," J. of Protective Coatings and Linings, August 1987, p.38.
17 D'Amico, E. "New Patch of Regulations to Come," Modern Paint and Coatings, April 1997, p. 22.
18 Greene, J. "Polysiloxanes for High Temperature Resistant Powder Coatings", Paint and Coatings Industry, February 1998.
19 US 6,344,520, 2002, J. Greene (Wacker Silicones Corporation).
20 Meilus, L. "Innovative Solutions for Anti Corrosive Protection Using New-Generation Coatings", Offshore Coatings Technology, Committee STG 02-Protective Coatings and Linings-Atmospheric and STG 03, Corrosion/2004 NACE Show, New Orleans, 3/2004.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




