Fast-Curing Oligomers for Enhanced Coating Performance

Formulators are often able to achieve increased cure rates using conventional free amine synergists such as methyl diethanol amine, dibutyl ethanol amine, triethyl amine and triethanol amine. These amines react synergistically with Type I hydrogen-abstracting photoinitiators such as benzophenone or isopropyl thioxanthone (ITX). Ultimately, they enable formulators to overcome oxygen inhibition, which negatively affects the surface cure of coatings.
However, conventional amine synergists present a number of formulating concerns. A key issue is odor, which can be a problem before, during and after UV curing. In addition, "blooming" of the amines to the surface may result in a grease-like film on the finished coating. Finally, conventional amine synergists may be a source of extractables in cured coatings.
The High-Performance Alternative: Acrylated Amine Oligomers
Innovative acrylated amine oligomers present a viable option to conventional amine synergists. They enable UV-cure formulators to increase the surface cure rate and overcome oxygen inhibition - like conventional amine synergists. But they also provide performance and processing benefits that conventional products do not, including the following.- Lower volatility
- Lower odor (before, during, and after curing)
- Lower film extractables
- Lower VOC formulations for simpler environmental compliance
- No blooming
- Improved color (less yellowing)
- Improved solvent resistance

Acrylated vs. Free Amine Synergists
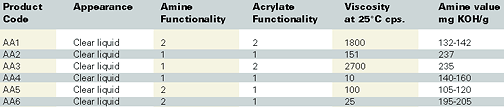
Recently, chemists from Sartomer Co. evaluated several amine synergists in a basic UV-curable formulation. They compared MDEA (methyldiethanol amine, a free amine control) with acrylated amine (AA) 1, AA2, AA3, AA4, AA5, and AA6. The acrylated amines are differentiated in Table 1.A formulation containing the workhorse epoxy acrylate oligomer and monomers commonly used in the UV-cure coatings industry was tested with a hydrogen-abstracting photoinitiator and varying amine synergists. These particular monomers were chosen for two reasons - they are among the most common in the industry, and they do not have any ethoxylation present in their backbones. (Ethoxylation further enhances the cure rate of UV-curable formulations, particularly when used with an amine synergist). Using these monomers enabled chemists to fully determine the affects of the amine synergists.
All formulations (see Table 2) were made into films using a number 5 wire-wound rod and cured with a 600 watt/inch Fusion H lamp in air at 50 feet/minute. Surface cure speed, MEK resistance, yellowing index and weight loss were tested for each formulation.
Results
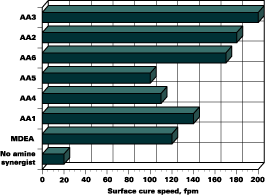
Surface Cure Speed
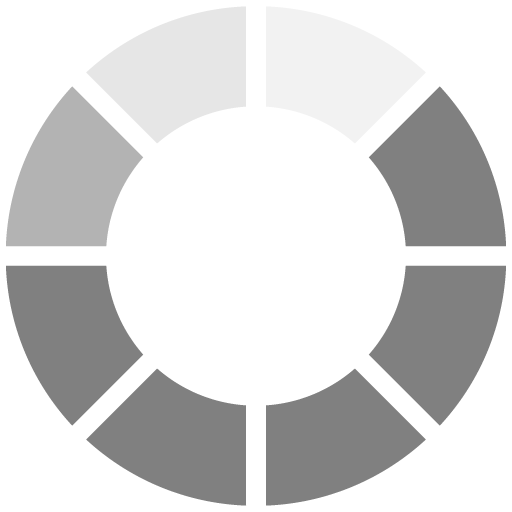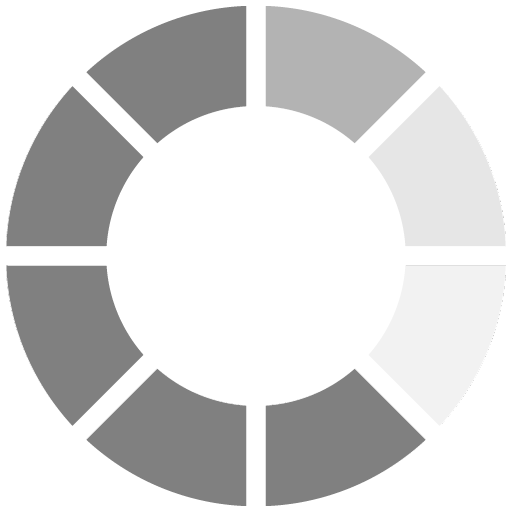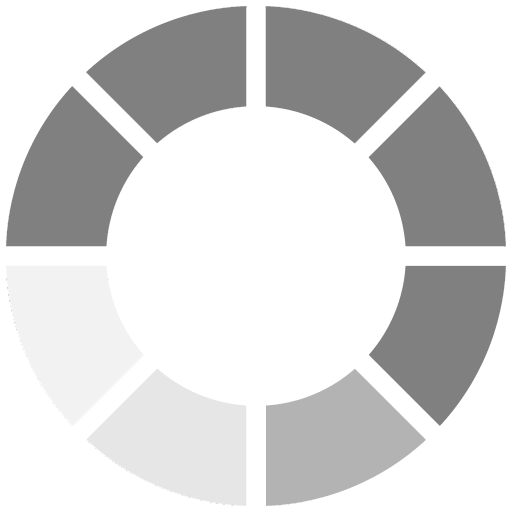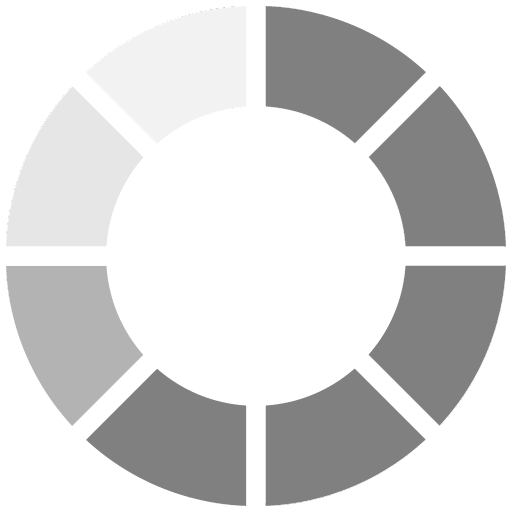
The surface cure speed of each film was tested using a fingernail scratch test. The significance of adding an amine synergist is apparent in Figure 1. The surface cure speed results reveal that AA3 and AA2 are the best performing acrylated amine synergists tested in the formulation. All of the amine synergists outperformed the conventional MDEA in terms of cure speed, with the exception of AA4 and AA5. 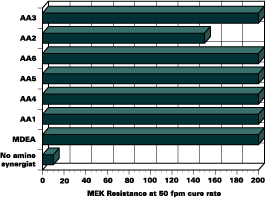
MEK Resistance
Methylethyl ketone (MEK) resistance was tested to determine the through-cure effectiveness. The films were cured at 50 feet/minute. A maximum of 200 double rubs was performed. Overall, the films containing no amine synergist faired poorly. All of the acrylated-amine formulations met the 200 double rub criteria except AA2. The dramatic increase in through cure with the use of an amine synergist is clearly demonstrated by the results presented in Figure 2. These results prove that coatings can be cured efficiently using acrylated amine synergists. More significant, the results show that acrylated amines increase cure rates without a loss in crosslinking efficiency.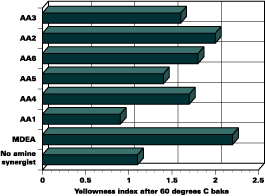
Yellowness Index
The yellowness index of clear and pigmented films is another important characteristic to consider when formulating UV-curable coatings. Free amines may discolor clear coatings, causing an undesirable yellow color.
The cured films in this study were analyzed after a 24 hour bake at 60 deg C in a forced air oven. All of the films containing acrylated amines performed better in terms of film yellowing than the films containing the free amine, MDEA (Figure 3).
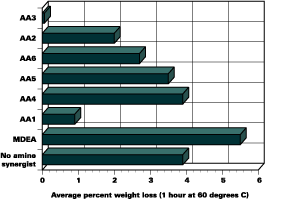
VOC Content
Film performance is only one concern for formulators. Environmental and safety issues must also be considered when formulating UV-curable coatings. Regulatory changes, as well as the health and safety of those working at press side or during the curing process, must also be considered. Specifically, the volatile organic compound (VOC) content of the formulations must be evaluated.For each formulation, the chemists determined weight loss after one hour at 60 deg C to reveal the VOC content. As Figure 4 shows, the acrylated amine synergists are very low in volatility. The resulting lower VOC content makes it easier for formulators to meet regulatory requirements and protect employees. Additionally, low VOC formulations enable them to provide a low-odor work environment and low-odor end products for customers.
Performance and Processing Advantages of Acrylated Amines
Based on the results of this study, the chemists determined that acrylated amines are superior alternatives to free amine synergists in UV-curable coatings. They enable faster surface cure speeds, while providing additional coating-performance advantages such as reduced volatility, enhanced yellowing resistance, and increased solvent resistance.The chemists also determined that acrylated amines provide processing and performance advantages during and after curing. Specifically, they result in lower volatility, lower odor (before, during, and after curing), lower film extractables, and lower VOC formulations, which do not result in blooming.
For more information on oligomers, contact Sartomer Co., Inc., 502 Thomas Jones Way, Exton, PA 19341; phone 800/SARTOMER or 610/363.4100; fax 610/363.4140; visit www.sartomer.com; or e-mail lujean.burak@sartomer.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!