Nano-Clays in Polyester Gelcoats
A type of smectite, montmorillonite, can absorb 20-30 times its volume in water, and is of particular interest to the plastics industry. It is a 2:1 layered structure; a single layer of aluminum octahedra sandwiched between two layers of silicon tetrahedra. Each layered sheet is slightly less than one nanometer, or 10?, with surface dimensions extending to about a micron (1,000 nanometers). With aspect ratios approaching 1,000, surface area of the clay is in the range of 750 square meters per gram. Due to the isomorphous substitution by magnesium and iron in the octahedral aluminum layer, a certain charge deficiency occurs at the sheet surface. This charge is typically satisfied by sodium and calcium cations. In its natural state, when hydrated at 8-10 wt%, the sheets are stacked on each other (due to surface attraction), and the spacing between each sheet, called the gallery, is normally 3-5A. Additional water molecules can readily enter the structure and coordinate with sodium cations, and expand the distance between the sheets.
Nano-Clay Preparation
The first step in the preparation technique of the nano-clay is the purification (up to 99%) of the mineral (clay). The second is the modification of the surface of the mineral to make it compatible with specific resin systems (thermoplastics or thermosets) because the minerals are generally hydrophilic and, therefore, relatively incompatible to the resins.
This stage is very similar to the treatment of fiberglass reinforcements with coupling agents (silanes) to ensure a perfect compatibility and even a chemical bonding with the resins they are intended to reinforce.
The most popular surface treatment for the clays is the use of organic ammonium cations, which can be exchanged for the inorganic cations existing on the silicate surface. Surface treatment reduces particle-particle attraction, promoting an expansion of the gallery to above 20A. The treated clay, now with an organophilic surface, can then be readily incorporated into the resin matrix. At this distance the particles can be separated further either by adsorbing monomer into the gallery prior to polymerization or, in the case of a high polymer, by using shearing force using an extrusion compounder. Although the initial delivered particle size is about 5 microns during dispersion in the resin matrix, the clay breaks down and reaches the desired nanometric dimensions.
a.) Intercalation, when the sodium ion is left on the surface, takes advantage of the dipole induced on the back side of the sodium which is attempting to neutralize the charge deficiency in the octahedral layer. The induced partial positive charge on the sodium can react with partial negative charges on functional monomer or polymer groups. These functional groups having negative dipole moments include alcohols, carbonyls, esters, amines and ethers (polyvinyl alcohol, polyvinyl pyrrolidone, polyethylene oxide, etc.).3 Depending on the organic molecule (or intercalant) used, the intercalant forms a unique and discrete complex with the clay surface, called a nanomer (chemically modified nano-clay).
b.) Coupling agents, which use organo-functional silanes as the treatment.4 In this case, the silanes will react with silanol groups present in the layered clay, mainly positioned at the edge of the clay layer.
Complete dispersion of the nanomers in the resin matrix is called exfoliation and the result is a nanocomposite product. With complete exfoliation, the maximum surface area is available for interaction between the clay and the polymer, and the polymer is essentially saturated at perhaps 5 wt%.
The commercial availability of compounded polymer nanocomposites was initially restricted to mostly polyamide polymers and thermoplastics. There are, however, studies available for epoxy and polyesters.2,5-6 The nanocomposites display the magnifying effect of combining aspect ratio and nanoscale size, not available with conventional composites. The particle-molecule interaction creates a constrained region at the particle surface, which immobilizes a much larger portion of the resin matrix (for example in the nylon resin systems, the constrained region exceeds 60% of the total matrix).
Even at low concentration of nano-clays, the fracture energy was doubled, the Young's modulus and heat distortion temperature were substantially increased, and the rheological properties were modified. Gas permeability and liquid absorption rates were tremendously reduced.5,7-9 Other benefits include fire/smoke control properties, superior barrier properties against UV, water and oxygen penetration and shrinkage control.10

Experimental
Usually the challenge in using fillers in any resin system may be the higher viscosity due to the thickening effect of the tiny particle size. All the information described above made us believe that the nano-clays might however find interesting applications in polyester gelcoats, due to the unique dimensions of the particles.The gelcoat is an important part in composites, for well established performance reasons.11 Generally they are specially formulated polyester resins with a thixotrope, catalysts, fillers, pigments and other additives to impart the desired process and performance properties for the whole composite. From the processing point of view, the principal properties of the gelcoat are the way in which the material flows and holds-up, possibly on a vertical mold surface.12 While these two effects appear to be mutually exclusive, most coatings must be formulated such that the rheology adequately accommodates both requirements.
Fumed silica, with a surface area of about 200 square meter per gram, is the most common additive used as a thixotrope and provides the required film thickness, determines the viscosity and improves nonsag, as well as the pumping characteristics of the gelcoat. Extensive investigations describe the rheological behavior of fumed silica in laminating resins and gelcoats, and the thixotropic effect of fumed silica is explained as an interaction of silanol groups by way of hydrogen bonds to form a three-dimensional chain structure that provides increased viscosity.13 Also, it is assumed that the fused particle chains of the fumed silica are at the same time responsible for the general gelcoat properties. During the mixing of the gelcoat, the shear forces break up the formed matrix, thus reducing viscosity, but the reformation of the hydrogen bonds takes place upon disruption of the shear forces. The acid number and hydroxyl value also have a tremendous impact on the rheological behavior of fumed silicas in gelcoats.
Materials
A modified acrylated gelcoat resin and a general purpose (i.e., conventional) unsaturated polyester resin for the core were used for the study. The nano-clay was an onium ion modified montmorillonite.14
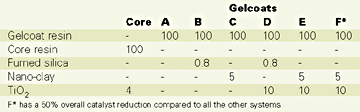
The gelcoats (A through F) and the core mix were formulated as shown in Table 1 expressed in phr (weight parts per 100 parts of neat resin). These formulations and the mixing conditions do not necessarily reflect practical requirements for the resin mixes.

Procedure
All the resin mix components were dispersed for 20 minutes on a mixer with a blade diameter of 50 mm. A shear rate of 7.8 m/sec (3,000 rpm) was applied, if not otherwise specified. All components were added together in the mixing container, under the same conditions. The same single composition was used in all cores for all panels.A film thickness of 0.3 mm of gelcoat was drawn down on a horizontal surface and then semi-cured in the convection type oven at 126.7?C for 20 minutes. A flat FRP panel structure (1,500 gram per square meter total weight and 1.37 mm thick) was then built together using the above gelcoat and the core, with 25 w% total glass content. The whole composition was cured in the convection type oven at 126.7?C for 25 minutes.
The reactivity of the gelcoat resin mix was evaluated by measuring the gel time and exotherm of 50g of material in a 100ml glass beaker. The test was conducted in a water bath at 82.2?C. The point at which the resin reaches 37.8?C is set as time equal to zero.
The gloss was measured on the gelcoat side of the FRP panel at 60? with a Byk-Gardner micro-TRI-gloss instrument, as per ASTM D 523-85 standard. The difference ("d" gloss) between the initial and the final (after exposure) gloss was reported.
The color on the gelcoat side of the panel was measured with a Dataflash 100 colorimeter from Datacolor International. The CIE system of L*, a*, b* values represents whiteness, redness and yellowness, respectively, with the corresponding complementary colors at the opposite end of each axis of 3-D colorspace. The color change, dE, was calculated after each exposure test.

In the water boil test, the panel samples were dropped into a glass container with boiling distilled water, which had been allowed to equilibrate for at least 15 minutes, and placed on a hot plate to maintain the boiling. During the testing the specimens must remain completely immersed in water. After a certain time, the samples were removed from water and inspected for any change in the surface appearance, such as gelcoat crazing or cracking.
The Emmaqua test is an accelerated outdoor weathering test performed in Arizona, where the solar radiation is magnified by a sun-tracking mirror device. A total radiant exposure energy of 280 MJ/m2, in accordance with ASTM G 90-94, Procedure B, and considered to be the equivalent of one year of real-life natural outdoor exposure in Arizona was applied on each panel surface. This procedure has a standard spray cycle that provides an eight-minute water spray once every hour during the daylight hours when the machine is operating, with an additional eight-minute water spray at 9PM, 12AM and 3AM. The specimens are mounted unbacked in an aluminum frame.
Xenon arc test exposure was performed as per ASE J1960/ASTM G 26 standards, with an Atlas Ci65-A instrument, with the following continuous cycle:
40 minutes light
20 minutes light + front spray
60 minutes light
60 minutes dark + back spray
Total 180 minutes / cycle.
The cycle conditions are:
Light Dark
Ambient temperature, ?C 73 3 37.8
Spray temperature, ?C 45.0 40.0
Relative humidity, % 50 95
The total radiant exposure energy for each panel sample was 1281 KJ/m2 at 340 nm wavelength.
UV exposure was performed with a QUV Accelerated Weathering Tester instrument from Q-Panel Co., with a combination of condensation and fluorescent UV, as per ASTM G 53. UV-313 B bulbs were used on the gelcoat side of the panels with the following cycle: 4 hours of UV at 60?C, followed by 4 hours of condensation at 50 deg C.
Heat exposure: two types of infrared radiation tests were performed. In the first test (conventional infrared), the panel samples, gelcoat face up, were exposed directly to a 100 W floodlight bulb positioned over the sample at a distance, such that a surface panel temperature of 137.8?C was achieved. After a certain time, the samples are removed, allowed to achieve 25?C and inspected for gelcoat cracking. In the second test (halogen infrared), a similar inspection was performed after the samples were exposed to a 1500 W quartz halogen bulb, such that a surface panel temperature of 148.9 deg C was achieved.
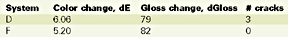
Results and Discussion
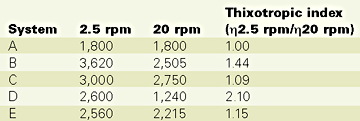
Table 2 summarizes the rheological results of the gelcoat mixes.Comparing both "B" with "C" and "D" with "E" it is obvious that the nano-clay does not generate the same thixotropic index as the fumed silica. This is possibly explained by the lack of particle chains in the nano-clay systems. Other types or different loads of nano-clays may however perform differently. Also, for some gelcoat processing conditions this new kind of thixotropic behavior might be satisfactory for certain industrial applications.
The gelcoat system "D" was used in making the Control panel. Table 3 illustrates the reactivities for the gelcoat mixes.
Using the same catalyst system as in the control (Gelcoat "D"), the surface-treated nano-clays in system "E" proved to act as a crosslinking activator. As a direct result, the gelling time and temperature were significantly reduced; the exotherm reaction started earlier, but its temperature was somehow reduced. The reactivity of the gelcoat was therefore adjusted to a reduced catalyst level (system "F") to better match the control. This adjustment was performed for both theoretical and practical reasons. On one hand, for the sake of this research, we didn't want the change in reactivity to play a significant role in the panel performance (which is normally the case for the unsaturated polyester resins). On the other hand, for processing reasons, such a reactive resin system would affect the pot life of the gelcoat. As seen, the exotherm for the system "F" is still lower, a sign of a lower degree of crosslinking in the polymer and, hence, a lower than expected performance of the panel. However, the nanocomposite panel produced with this latter formulation and evaluated against the control, had a better overall performance. The water boil test results are presented in Table 4.
After an hour of boiling, the control displayed 50 surface cracks. The exposure of the nanocomposite panel was stopped after four hours of continuous boiling, without any gelcoat cracking. This superior performance is explained by the presence of the nano-clay shield barrier at the gelcoat surface. The results for the Emmaqua exposure test are presented in Table 5.
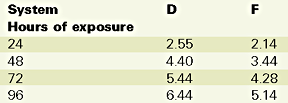
This phenomenon is called chalking.15 It is also known that the chemical reaction responsible for chalking is the oxidation.16 A reduced chalking should be interpreted as a reduced oxygen penetration at the superficial surface level, due to the presence of the nano-clay in the gelcoat. The results of the Xenon arc exposure are presented in Table 6.
Based on these results, there is no longer a significant difference between systems. However, there is a noticeable 15% better color stability for the nanocomposite and a slight better gloss retention for the control. The difference between the Emmaqua and the Xenon arc test results should be found in the difference of the test conditions, i.e. in the latter the main reason for "weathering" is no longer the UV, but heat and moisture, followed by the UV radiation. Table 7 illustrates the QUV test results.
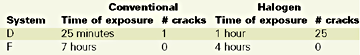
In the first test, the control panel cracked after 25 minutes when exposed to the conventional infrared exposure, while the nanocomposite ("F") was exposed for a full seven hours without cracking. In the second test, 25 cracks have developed on the gelcoat surface of the control, while the nanocomposite panel did not crack after four hours of halogen lamp exposure.
Conclusion
In the unsaturated polyester resin researched systems, the nano-clay did not work as effectively as a thixotropic agent as the fumed silica (but rather, as a thickener), but may be acceptable for certain gelcoat applications and/or in combination with fumed silica. This particular nano-clay acted as an activator for the catalyzed gelcoat resin, speeding up the gelling and the exotherm reaction. The reactivity curve can probably be further adjusted, but this was not the purpose of this research. In spite of a lower exotherm temperature in the gelcoat, all the nanocomposite panel properties tested proved to be better than the control. The heat-related crack resistance of the gelcoated FRP panel was the most impressive performance, followed by better color stability under UV radiation.For more information on nano-clays, contact Lasco Composites LP, 8015 Dixon Dr., Florence, KY 41042; phone 859/371.7720; fax 859/371.1913; e-mail ccapanescu@lascocomposites.com; or Circle Number 133.
Acknowledgements
The authors wish to thank Mr. Jim Amundson, president of Lasco Composites, for his enthusiastic support, and to Professor Mihai Dimonie and the whole Department of Macromolecular Chemistry of the University Politehnica of Bucharest for the permanent scientific guidance.
Also, we are grateful to the Nanocor Inc. team, especially to Dr. Tie Lan and Ron Gartner, for their assistance.
References
1 S.Shelley, Chemical Engineering, (July,1999)38.2 T.Lan, Nanocor, Inc., private communication (1999).
3 U.S.Pat. 5,552,469 (Sep 3,1996) G.Beall, S.Tsipsursky, A.Sorokin, and A.Goldman.
4 U.S.Pat. 5,910,523 (June 8,1999) S.Hudson.
5 X.Kornmann, L.A.Berglung, J.Sterte, and E.P.Giannelis, Pol. Eng. and Sci, vol.38, no 8, (Aug 19, 1998)1351.
6 R.Dagani, Science/Technology, (June 7,1999)25.
7 J.D.Destefani, Molding Systems, (Oct,1999)32.
8 R.Krishnamorti and E.P.Giannelis, Macromoleculaes, (1997)30, 4097-4102.
9 T.Pinnavaia, T.Lan, P.D.Kaviratna, Z.Wang, and H.Shi, Pol.Mat.Sci. and Eng.Proc., 73, (1995)117.
10 L.M.Sherman, Plastics Technology, (June,1999)54.
11 C.Nistor, S.Ripszky, and Gh.Izrael, Materiale Termorigide Armate, Ed.Tech, Bucuresti, (1980)108.
12 P.A.Reynolds, Rheology of Coatings, Royal Soc. Of Chem., Cambridge, (1994)42.
13 J.Hoell, Soc. Plastics Ind., (1977) 32, 17-D.
14 Supplier, Nanocor Inc. sales literature "Nanomer I.28E", (1999).
15 Plastic Design Library, The Effect of UV Light and Weather, chapter 27, NY, (1994)165.
16 L.C.Delre, Engineering Plastics, Vol. II, (1988)575-580.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!