Acrylic Dispersions for Industrial Coatings with Polymer-Bound Stabilizers
Recent changes in the regulations concerning the emission of organic solvents in the European community have led to a growing interest in the use of polymer dispersions in high-performance waterborne coatings systems. One-pack systems, using the current state-of-the-art technology of combining carbonyl-hydrazide crosslinking with control of the morphology of the particles in the polymeric dispersion, have been studied extensively.1,2 These acrylic dispersions are synthesized using conventional surfactants as an integral part of the recipe. Even though some grafting might occur during the polymerization, the surfactants are predominantly adsorbed onto the surface of the polymer particles.3
The presence of surfactant has an influence on the final paint film properties. During film-formation, coalescence is caused by self-diffusion and results in polymer interpenetration. This process requires the disappearance of surfactant from the surface of the polymer particles. Because most surfactants are not fully compatible with the polymer matrix, surfactant will have the tendency to migrate to the film-air interface. Therefore, the surface structure of a film cast from a dispersion will be different from its internal structure. Because of the non-volatile nature of the surfactant, a permanent reduction of surface hardness is observed. In coatings, this effect can lead to bad blocking resistance, inferior sanding properties and dirt pickup. In some cases, the surfactant is completely exuded from the film. This effect was observed by Hellgren et al4 using atomic force microscopy. As a result, resin chemists have been actively pursuing alternatives for conventional low-molecular-weight surfactants.

Surface-Active Monomers
A possible way to overcome these problems is to covalently incorporate the surfactants in the polymer chain so they become ‘anchored' to the particles. This requires surfactants that possess a ‘reactive' group that is active through a free radical mechanism. A simple maleate surface-active monomer, or ‘surfmer,' has been described as surfactant in the emulsion copolymerization of styrene and of styrene/butyl acrylate/acrylic acid.5 This surfmer has been investigated in the preparation of self-crosslinking acrylic dispersions.6 The use of this surfmer resulted in improved coating properties, such as better gloss retention, water-barrier properties and blocking resistance. At present, both in-house or commercially made surfmers are available. An essential feature for a surfmer is a double bond with a relatively low reactivity. If the copolymerization with (meth)acrylic or styrene monomers is too fast, the surfmer will become buried within the polymer particle and hence lose its stabilizing function. The use of surfmers is especially important in the replacement of alkyl phenol-based surfactants (APEO). It is well known that the currently available alternatives, mainly based on fatty alcohol hydrophobes, are less compatible with the polymer matrix than the APEO-types and will therefore be more prone to migration. This results in a significant reduction in water resistance as illustrated in Figure 1 where polymer dispersions with identical monomer compositions but prepared with different surfactants are shown after water-soaking.
Although surfmers offer a viable alternative for conventional surfactants and solve many of the related coating problems, an important concern is to get complete conversion of the surfmer. Conversions for the maleic types have been reported to be in the range of 60 - 70%. Allylic types have conversion of about 80 - 90%. This means that some migration of non-reacted surfmer still may occur.

Polymeric Stabilizers
Another route to circumvent the use of low-molecular-weight surfactants is the use of polymeric stabilizers in the emulsion polymerization process. Polymeric stabilizers have been used for a long time.7 Typically, the polymers used are styrene/acrylic acid resins prepared by solution or bulk polymerization in the presence of a chain transfer agent, having an acid value of 140 - 300 mg KOH/g and a molecular weight of 1000 - 5000. The problem associated with the use of these high-acid-value polymeric stabilizers is the intrinsic water sensitivity of coatings made from these dispersions. Nuplex Resins has developed a polymerization process in which we can make polymeric stabilizers with an acid number that is considerably lower than the ones commonly used.8 Furthermore, carbonyl groups are introduced in the polymer so the stabilizer can co-react with a hydrazide crosslinker (Figure 2).
In this way, the stabilizing polymer gets built into the network formed by the carbonyl-hydrazide self-crosslinking reaction. The stabilizers can be used in an emulsion polymerization process to obtain polymer-stabilized polymer dispersions (Figure 3).

Synthesis of a Polymer-Stabilized Acrylic Dispersion
A polymeric stabilizer was synthesized by copolymerization of methyl methacrylate, n-butyl methacrylate, methacrylic acid and diacetone acrylamide. Some of the properties of the polymeric stabilizer used are given in Table 1.After (partial) neutralization with ammonia, the polymeric stabilizer is dissolved in water. The solution is clear and slightly viscous and is used in the emulsion polymerization of methyl methacrylate and n-butyl acrylate. The monomer ratio used in the polymerization is given in Table 2.
In addition to the main monomers, carbonyl functionality was introduced using diacetone acrylamide. The ratio between the polymeric stabilizer and the second-stage polymer was 1:1. The polymerization conditions (temperature, dosing time, monomers etc.) were optimized to maximize grafting reactions between the stabilizer and the second-stage polymer. The resulting polymer dispersion is nearly translucent, an indication of a mono-modal, small particle size distribution. The properties of the dispersion are given in Table 3.
A low minimal film-formation temperature (MFT) characterizes the dispersion even though it contains a high percentage of the hard polymeric stabilizer. This can be attributed to the occurrence of hydro-plastization. Hydro-plastization means that water is retained in the drying coating for a longer period of time and that it effectively reduces the MFT of the dispersion. Once the neutralizing base has evaporated from the dispersion film, the effect disappears and a hard hydrophobic polymer remains.
Gel permeation chromatography (GPC) was used to estimate the amount of polymeric stabilizer that had become grafted onto the dispersion particles. Figure 4 shows the chromatogram of the polymeric stabilizer (left) and of the end product (right). From the shape of peak 1 in Figure 4B, it is clear that the molecular weight of the second-stage polymer is above the exclusion limit of the GPC-columns used.
From the chromatograms we can conclude that a substantial part of the stabilizer is covalently grafted to the second-stage polymer. Ultra-centrifugation of the diluted dispersion was used to quantify the percentage that was grafted. It was found that about 50% of the polymeric stabilizer used was recovered in the supernatant.
Paint Evaluation
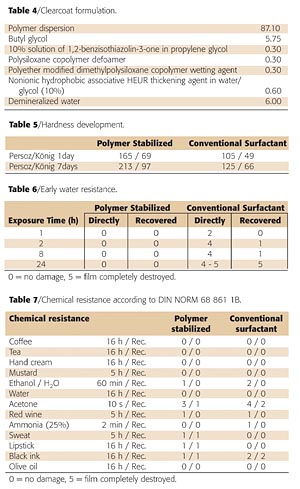
A simple clearcoat formulation was made with the polymer-stabilized acrylic dispersion. The formulation is given in Table 4.The mechanical properties of the clearcoat were investigated by means of dynamical mechanical thermal analysis (DMTA). Figure 5 shows a DMTA plot of a film dried at ambient temperature for seven days.
The plot of the loss tangent (tan D) as a function of temperature clearly indicates that two glass transitions are present in the film. The first broad transition at a temperature of 44 deg_C can be attributed to the crosslinked second-stage polymer. A second, sharper transition can be observed at a temperature of about 130 deg_C. This transition must be attributed to the crosslinked polymeric stabilizer.
We compared the paint properties of the polymer-stabilized dispersion with a commercially available dispersion stabilized with a conventional surfactant and with a similar MFT. Table 5 compares the hardness development.

Even though the polymer-stabilized dispersions contain a relatively large amount of hydrophilic stabilizing polymer, the carbonyl-hydrazide crosslinking ensures that the water sensitivity is minimal. This is even more clearly illustrated in Figure 6 where two panels are shown immediately after a water immersion test performed at 50 deg_C after 16 hours of film drying at ambient temperature.
The chemical resistance properties of the clearcoats were checked according to DIN 68861 1B after one week of ambient temperature drying (Table 7). To make the test more stringent, the spots were also judged immediately after removing the stain.
Even though the new polymer-stabilized dispersions meet most of the requirements for the industrial coatings market, improvements still can be made when it comes to the water resistance only hours after the application of the coating. Also, the chemical resistance against aggressive substances at elevated temperature (hand cream, suntan lotion, bug spray) can be improved.
The fact that these properties are not sufficient yet can be explained by the fact that when the carbonyl-hydrazide crosslinking reaction has not yet reached full conversion, the non-grafted polymeric stabilizer may be partially extracted from the film.

Macromer Stabilizers
It is to be expected that the degree of attachment of the polymeric stabilizer will be increased by the introduction of a copolymerizable ethylenical unsaturation at one end of the polymer chain. Such a polymer is often referred to as a macromonomer or macromer. A macromer stabilizer can be used in an emulsion polymerization in the same way as the polymeric stabilizers described above.
A proprietary polymerization process has been developed by Nuplex Resins to make macromer stabilizers using an addition fragmentation transfer (AFT) agent that does not involve the use of heavy metals. The general structure of the AFT agent used is given in Figure 7. The AFT agent can react with a propagating polymer chain as described in Figure 8.
The addition product formed between the propagating polymer chain and the AFT agent contains a tertiary free radical and can fragment back either to the original state or as indicated in Figure 8. In the latter case, the initial propagating chain is terminated with a double bond. The radical fragment can initiate a new polymer chain. We have found that this reaction produces macromers when methacrylic monomers are mainly used.
Using this technique, we prepared a macromer for use as a stabilizer by copolymerizing methacrylic acid, butyl methacrylate, diacetone acrylamide and methyl methacrylate in the presence of the AFT agent. The final molecular weight of the macromer is determined by the ratio of the initial concentrations of monomer and AFT agent. The polymerization was carried out in methoxy propanol with tert-amyl peroxy-2-ethylhexanoate as initiator. The macromer was isolated and the molecular weight was determined using GPC. The chromatogram is given in Figure 9.

The macromer stabilizer was used as a substitute for the polymeric stabilizer in the emulsion polymerization process described above. The resulting polymer dispersion had a fine particle size of 70 nm and was free of coagulum, demonstrating that the use of a macromer stabilizer does not significantly change the nucleation and particle growth process. To determine the effect of the terminal double bond on the degree of grafting of the stabilizer, the dispersions were diluted and ultra-centrifuged. It took more than 100 hours at 30,000 rpm before a clear supernatant could be observed. By determination of the solids content of the supernatant, we found that the concentration of water-soluble material in the dispersion was extremely low (less than 1%). NMR analysis of the water-soluble material revealed that this polymer was no macromer, indicating that the grafting efficiency of the macromer stabilizer is close to 100%. This is a significant improvement over the degree of grafting found with a stabilizer without terminal double bond. As we expected this to have a strong effect on the early water resistance, we repeated the water-soaking test as described above. The results, immediately after removal of the panels from the water tank, are illustrated in Figure 11.
The determination of the other paint properties is still ongoing.

Conclusions
It has been demonstrated that replacing conventional low-molecular-weight surfactants by stabilizers that can be covalently bound to the main polymer results in polymeric binders that can be used in the formulation of coatings with improved properties. Acrylic dispersions using surfmers or polymeric stabilizers are now being fully commercialized. The macromer stabilizer route seems to hold promise for further improvements in the near future.Acknowledgements
The author would like to thank Anneke van der Zande for the polymer synthesis and Theo Klijn for the paint evaluation work. Martin Bosma of Akzo Nobel Chemicals Research Arnhem performed the DMTA analyses used in this paper.
A modified version of this paper was published in PPCJ, June 2004.
For more information, contact Nuplex Resins BV, the Netherlands; e-mail Dirk.Mestach@NuplexResins.com.
References
1 Mestach, Polym. Paint Col. J, Vol 190 No 4431: 28, 2000.2 Mestach, Loos, Proc. XXIV FATIPEC Congress, Vol B: 91, 1998.
3 Fitch, McCarvill, J. Colloid Interface Sci.Vol 66 No 1: 20, 1978.
4 Hellgren, Weissenborn, Holmberg, Progr. Org. Coat., Vol 35, 1-4: 79, 1999.
5 Sindt et al., J. Appl. Polym. Sci. 2000, Vol 77 No 12, 2768-76.
6 Mestach, Progress in Colloid and Polymer Science, 124 : 1-5, 2004.
7 British Patent GB-A-1,107,249 to S.C. Johnson.
8 de Krom, Mulder, Mestach, Proceedings of the NPIRI Technical Conference, October 2000.
9 Amick et al., U.S. Patent 5,314,977 to Rohm and Haas Company.
10 Webster, in Living Polymerization Methods, 251 Science 887 (22 Feb. 1991).
11 Haddleton et al., U.S. Patent 6,017,992 to Zeneca Resins.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





