The Effect of Water Resistance on the Durability of Waterborne Coatings

Waterborne coatings are especially susceptible to durability issues pertaining to poor water resistance. Most formulation components for waterborne coatings are either water soluble or have colloid stability (e.g., latex polymer). In all cases, the functional groups on polymers that are used are susceptible to hydrogen bonding or are ionic. Unless the hydrophilic character is balanced with the hydrophobic, the coating will either be water sensitive or the formulation will not have colloidal stability. In addition, the water sensitivity of the latex polymer binder may also impact overall coating water sensitivity. We have used coating water absorption, water vapor permeability and blister resistance to characterize the factors in waterborne coating formulations that pertain to water sensitivity. The factors we studied include: formulation components for stability and rheology control, as well as latex polymer hydrophobicity.
Our research shows that waterborne coatings can be made resistant to water and durable to ponded water situations such as those that might be encountered on low-slope roofs. We have found that hydrophobic components in the formulation, as well as the use of hydrophobic binders, will give the best combination for improving the water resistance of waterborne coatings. This will result in waterborne coatings that can resist blistering over hydrophobic substrates for up to four to six months of continuous immersion in water. However, in the design of polymers for ultimate durability, the UV resistance of the hydrophobic materials must also be considered to give the best exterior durability.
Experimental Techniques
Formulations
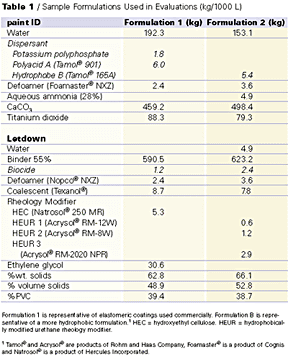
We focused our experimental measurements on formulations that are representative of those used commercially for elastomeric roof coatings. Sample formulations are shown in Table 1. These formulations are about 39% PVC (pigment volume concentration), composed of a small amount of TiO2 (3 to 5% PVC), with the rest being CaCO3 (about 35% PVC). The binder and pigment are formulated to about 50% volume solids.
While the formulations shown in Table 1 have some variability in volume solids and PVC, all experimental series were usually compared with the same PVC and volume solids. While the total levels of HEC and HEUR type rheology modifiers are similar (5.3kg compared with 4.7kg respectively), HEUR levels are significantly lower since all the HEUR rheology modifiers are 20% solids materials. The level of HEUR rheology modifier used was about one-fifth that of the HEC thickener to achieve the same Stormer viscosity.

Substrates
We chose a hydrophobic substrate, modified bitumen roofing material, to which the coating had low adhesion in order to enhance the blistering tendency of the coating. The particular bitumen used was modified with atactic polypropylene (APP) (US Intec Brai SP4). Adhesion under wet conditions was typically about 30-50 N/m for the formulation studies presented below. Unavoidably, some compositional changes in the latex polymer to alter hydrophobicity also increased adhesion to 290 N/m. This makes the effect of blister resistance confounded by both good adhesion and hydrophobicity. We have some evidence that supports the independent contributions of both hydrophobicity and adhesion.
Measurements
We conducted three basic measures of water resistance. Our first measurement on a "free-film" was made by measuring the weight of water absorbed as a function of time. The water absorption profiles of different coatings were compared. Another measure of water sensitivity was the permeability to water vapor (water vapor permeance). We used a variation of wet cup method ASTM D 1653-93 by flipping the perm cup and having the water in contact with the coating. Another measure was direct observation of blisters formed after immersion in water as a function of time. These lab-measured blistering numbers were usually compared after two weeks, but for some systems where little blistering was observed, the immersion time was extended. Degree of blistering was determined on a visual comparative rating scale. This scale was two dimensional, based on ASTM D 714. One degree was the density of blisters, which could range from F (few) to M (medium) to D (dense). Another degree of measurement was the size of the blisters. Both measures of blistering were compared to photographic standards. To determine statistically significant differences in subjective measures like blistering, we used the method of pair-wise comparisons. Finally, we also put out exposures in a trough as shown in Figure 1, and kept the water at the bottom filled continuously during the exposure period.
Results
We examined four aspects of water resistance in relationship to exterior durability: water absorption or swelling, water vapor permeance, blister resistance (laboratory measurement) and exterior blister resistance. Using these four measures we examined the effect of formulation additives on water resistance, as well as the effect of different binder hydrophobicity (as determined by solubility parameter calculations). These four measures of water resistance all showed consistent results. Exterior durability, as observed by the blistering troughs, can be improved significantly by using hydrophobic formulation additives and hydrophobic binders. Exterior blister resistance improved from one week to four months through a combination of hydrophobic formulation additives and hydrophobic binders. We also believe that increased adhesion contributes to improved blister resistance, but we were not fully able to deconvolute improved adhesion and increased binder hydrophobicity.
Effect of Coating Components on Water Absorption
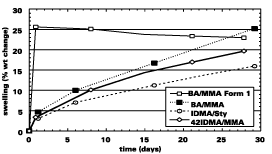
Figure 2 shows the amount of water swelling or water uptake (weight increase) that occurs for Formulation 1 with a butyl acrylate/methyl methacrylate latex polymer (BA/MMA). The water absorption of a standard elastomeric formulation with BA/MMA binder reaches its peak absorption of 26% within one day. On the other hand, when we use a more hydrophobic formulation (2), the water absorption reaches only 5% in one day. However, after four weeks the water absorption increased to the same level as that observed initially with the standard elastomeric formulation (25%). This may indicate enhanced initial water resistance, but if the coating is continuously exposed to water the effect will be the same.

Effect of Coating Components on Lab Blistering Resistance
We have performed several experiments that confirm the sensitivity of blister resistance to formulation additives. Thickener level is most important, while other factors (dispersant type and surfactant level) become more important as binder adhesion decreases. Table 3 shows the effect of changing the dispersant type from polyacid to hydrophobic copolymer, and the effect of changing thickener from HEC (higher level) to HEUR (lower level) on coating blistering. The coatings containing both hydrophobic copolymer dispersant and HEUR rheology modifier had the least blistering, while those containing HEC thickener and polyacid dispersant had the most.
As seen in Table 3, the effect of using a hydrophobic copolymer dispersant along with a HEUR thickener (due to low level of rheology modifier required for thickening) produces significant improvement in laboratory measures of blistering. This is seen in the differences in blister ratings and confirmed by the large difference in pair-wise scores. However, once the formulation changes are made there is little difference in the laboratory blistering measurements when polymers with different hydrophobicity are used. Table 4 shows that we see little difference in blister rating or score after two week's continuous immersion, and there is little change when the immersion time is extended to four weeks.

Effect of Hydrophobicity on Exterior Blister Resistance
Continuous immersion under ponded water conditions during the summer is the most severe test of blistering. Two separate exposures in troughs confirm lab results and show increased blister resistance of the hydrophobic formulation. Figure 3 shows that it takes about one week to blister a coating similar to formulations used commercially (formulation 1), and eight weeks to cause delamination.We can improve the time to initial blistering (but not the delamination time) by using the hydrophobic formulation 2 with BA/MMA copolymer binder containing HEUR rheology modifiers, as well as a hydrophobic copolymer dispersant. For the formulation change suggested (Table 1), the time to blistering onset increases from one week to four weeks (BA/MMA). However, complete delamination occurs in eight weeks, which is about the same as the standard formulation. This result is similar to that for water absorption presented previously. When the coating formulation is changed from #1 to #2, the initial water absorption is lowered, but after four weeks exposure the water absorption is the same.
The blister resistance of the coating is improved further by using more hydrophobic copolymers with long-chain methacrylates. Blistering resistance of coatings containing isodecyl methacrylate is significantly improved over that of BA/MMA copolymers (similar results are seen for copolymers of lauryl methacrylate). We can improve the blister resistance further by using styrenated versions (Figure 3). For instance, it took nearly four months for the IDMA/Sty copolymer coating to blister. We believe this is due to the lower water absorption of styrenated versions compared with MMA versions (12-15% swelling at four weeks versus 20-25% swelling, respectively). Similar resistance to exterior blistering resistance is also seen for lauryl methacrylate/methyl methacrylate binders as for IDMA/MMA binders (Figure 4).
The data we have presented shows that the formulation can strongly influence coating blister resistance, but that differences in polymer hydrophobicity may extend the blister resistance further. However, we have found that adhesion to the substrate may also be contributing to blister resistance. We have found previously that a binder with increased hydrophobicity will have greater adhesion to bituminous substrates, such as modified bitumen.2 Table 5 shows how blistering onset, wet adhesion and the calculated hydrogen-bonding component of the solubility parameter vary as the polymer composition is varied. First, we see that the wet adhesion for the coatings containing long-chain methacrylates is significantly greater than for a butyl acrylate/methyl methacrylate copolymer. However, if wet adhesion to modified bitumen were the only controlling factor in blister resistance, we would expect the coating containing 40 LMA to be almost as blister resistant as the coating containing IDMA/Sty, and this is not what was observed. The data seem to indicate that blister resistance associates closely with the hydrogen-bonding component of the solubility parameter. While the data seem to support increased hydrophobicity as the major contributor to blistering resistance, our data supports that both hydrophobicity as well as adhesive strength to the substrate ultimately determine blister resistance. Additional exposure data shows that 100% acrylate systems with HEC thickeners have blister resistance similar to that seen above with HEUR thickeners.
The results can be used to design a coating to meet various demands. In the case of elastomeric roof coatings, exterior exposure durability is very important. Therefore, UV resistance and resistance to damage due to thermal cycling need to also be considered in the design of a durable coating. While copolymer binders of long-chain (meth)acrylates and styrene give the best water resistance, they are also much more susceptible to UV damage (Figure 5).
Conclusions
The results presented above show that hydrophobic waterborne coatings could be formulated to have good resistance to blistering on exterior exposure. Hydrophobic copolymer dispersants, low levels of water-soluble thickeners and hydrophobic polymers will contribute to low water sensitivity. In addition, previous studies have shown the importance of adequate adhesion to the substrate. These data suggest that both good adhesion and low water sensitivity are needed to result in waterborne coatings that can resist blistering on continuous immersion for up to four months. While water resistance is important to the durability of coatings, particularly those exposed to ponded situations, it is just one factor in coating design, and the needs for a water-resistant coating need to be balanced against those that provide good UV durability. In this case, we found long-chain methacrylate polymers to provide the best balance of durability.References
1 Tamol and Acrysol are products of Rohm and Haas Company, Foamaster(r) is a product of Cognis, and Natrosol is a product of Hercules Incorporated.2 Kelly, DG; Yang, H.; Elfring, WH; Falcione, G; and Lau, W; Polymeric Materials Science & Engineering 85 (2001), 264-265, proceedings.
For more information, contact David Kelly, Rohm and Haas Co., at davidkelly@ rohmhaas.com.
This paper was presented at the 7th Nurnberg Congress, European Coatings Show, April 2003, Nurnberg, Germany.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



