Creating Better Automotive Refinish Coatings
with Cellulose Acetate

There are currently around 280 million registered vehicles in the United States, with estimates that this number will continue to rise. As more vehicles get on the road, there will inevitably be more vehicles that require refinishing due to wear and tear, and collisions. Therefore, modern automotive refinish formulations are designed to flawlessly restore visual aesthetics and cure as quickly as possible to ensure fast turnaround times. Like many other coating formulations, automotive refinish formulations have evolved over the years to be formulated primarily as water-based and low-VOC systems due to increased awareness and regulatory pressure surrounding environmental sustainability and public health.
Table 1 includes some common automotive refinish coating systems on the market today, along with key performance requirements that the market demands.
TABLE 1 | Common automotive refinish systems and performance requirements.
CAB = cellulose acetate butyrate, TPA = thermoplastic acrylic, HDI = hexamethylene diisocyanate
Many of the coating performance requirements listed above stem from the automotive refinish process. For example, since most automotive refinish coatings are applied by independent auto body shops and repair centers, which do not have robust drying equipment that OEMs have access to, refinish coatings must be formulated to facilitate faster solvent evaporation or quicker hardness development at relatively low temperatures. This is also necessary because parts being refinished may contain heat-sensitive assemblies.
Cellulose acetate butyrate (CAB) is a key ingredient that the formulations above include as a binder or additive to improve throughput and appearance and offer compatibility with low-VOC systems. First developed in the 1930s, these plant-based products have become an integral part of many coating formulations that prioritize productivity, appearance and sustainability.
How Do CABs Function in Refinish Coating Formulations?
CABs enrich automotive coating formulations by modifying rheology, reducing surface tension, having a relatively high Tg and rapidly building viscosity upon curing. These properties help form smoother films with improved dry-to-touch times, optimized visual quality and higher durability.
The rheology-modifying properties of CABs and their relatively high Tg create faster dry-to-touch times in basecoats and topcoats by effectively thickening the film and giving it a higher apparent hardness. This enables the polishing steps to be done sooner and makes the coating feel dry quicker and less likely to pick up dust and dirt while the solvent evaporates. In basecoats, CABs help modify rheology that allows metallic pigments to orient themselves to provide maximum reflectance and enhance metallic appearance. In both basecoats and topcoats, CABs enhance visual quality by imparting better flow and leveling, and reducing surface tension, thereby eliminating surface defects. They also enhance durability owing to the chemical structure of the CAB polymer, which imparts high resistance to weathering and yellowing. CABs also prevent solvent migration and redissolution from topcoats, better preserving color and appearance of the basecoat. For modern coating formulations that are based on waterborne and low-VOC technology, specific CABs have been developed for solubility in water/solvent blends and low- and VOC-exempt solvents.
To summarize, body shops and refinish formulations gain the following benefits from CABs.
- Quicker turnaround from faster dry-to-touch times.
- Improved appearance and reduced surface defects.
- Higher basecoat color consistency via resistance to basecoat redissolution.
- Compatibility with low-VOC formulations.
The following sections examine these benefits in further depth, with data quantifying performance improvements.
Faster Dry-To-Touch Times and Higher Early Hardness
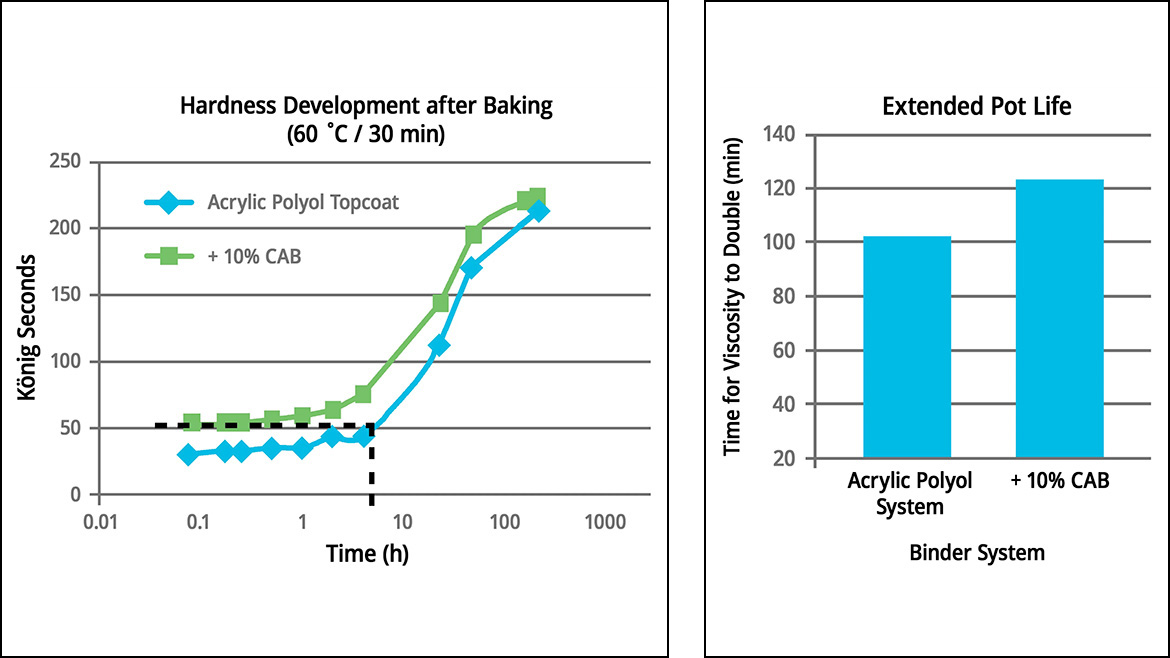
As mentioned, curing refinish coatings is relatively constrained compared to OEM coatings because body shops cannot use high heat to speed up evaporative cure. Thus, refinish coatings must be formulated to dry quicker to allow more refinish jobs to be handled per day. CABs facilitate a rapid rise in viscosity when a coating system is cured, creating fast dry-to-touch times that enable polishing steps to be done sooner. Figure 1 data shows that an acrylic polyol-based topcoat that included a CAB reduced sand-free drying time by 25% compared to the same system without the CAB. The drying time established with CAB was also 33% faster than industry standards.

CABs also help develop higher hardness early on during curing, which helps provide better mar resistance. The data in Figure 2 compares the previous acrylic polyol topcoat systems, one with 10% CAB and one without. Here, the topcoat containing the CAB developed a higher hardness immediately after baking at 60 °C for 30 minutes than the topcoat without the CAB, even after eight hours. Adding the CAB also extended the pot life of the topcoat.
Improved Appearance
Apart from protecting a vehicle’s material of construction, one of the main functions of automotive refinishes is to restore or improve the vehicle’s aesthetics. CABs function as flow and leveling additives in automotive refinish basecoats and topcoats to reduce surface defects and unevenness while increasing gloss and distinctness of image. Test panels of topcoat formulations were prepared with and without CAB, as shown in Figure 3. From a macroscopic observation, the topcoats containing CAB created a much smoother appearance. The red test panels illustrated the increase in distinctness of image that CABs provide, an important visual quality tied to the perceived value of the automobile.
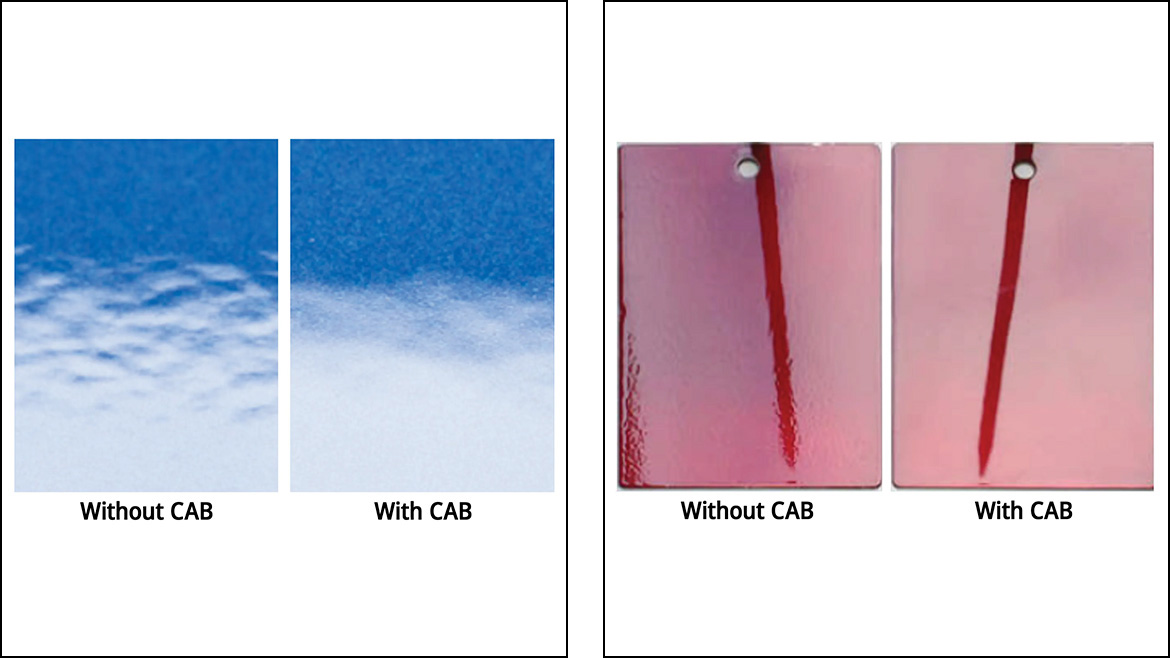
As body shops have relatively fewer automated processes than OEMs, there may also be a higher risk of surface contamination to contribute to surface defects that inhibit good-quality finishes. CAB's ability to improve leveling helps overcome coating defects that arise from surface contamination. The images in Figure 4 depict automotive substrates that were contaminated with WD-40. Coating formulations with and without CAB were applied to these substrates, where the formulation containing CAB yielded a significantly better appearance, as it resisted developing surface defects due to contamination.

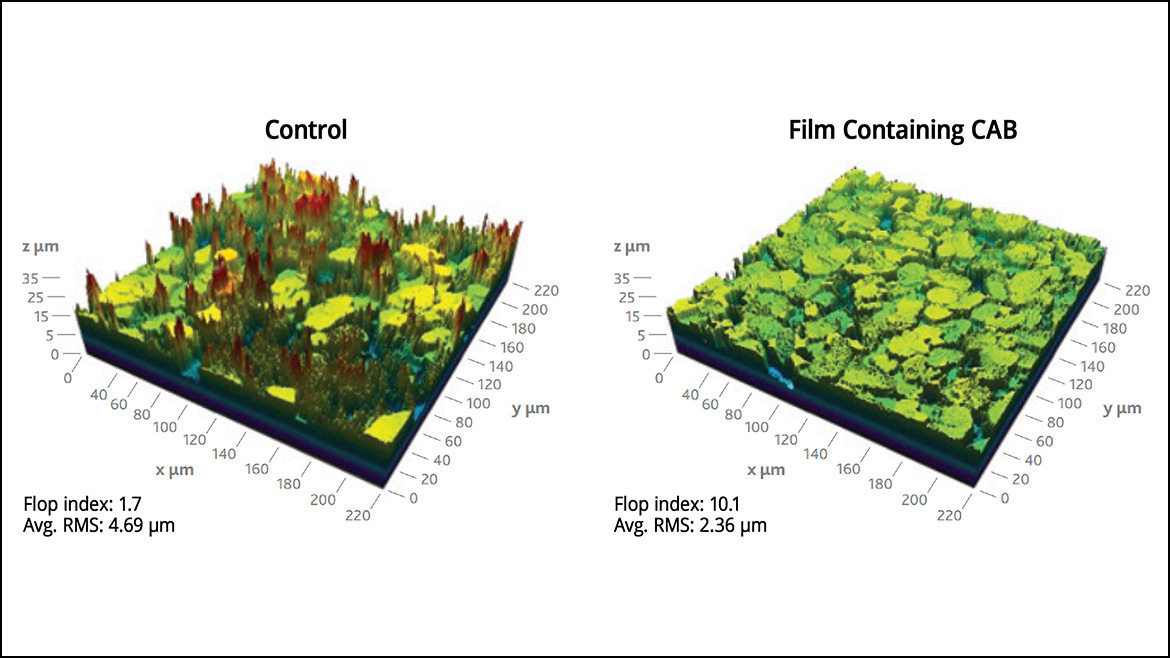
Visual quality also stems from the effect that metallic pigments provide in automotive basecoats. Metallic pigments are responsible for inducing a perceived change in color depending on the viewing angle, known as the flop effect. They also maximize light reflection, increasing the brilliance and attractiveness of the coating. When CABs are included in basecoat formulations, they help orient the metallic pigment flakes in angles consistent with a higher flop index and are optimized to reflect more light. The data in Figure 5 represents microscopic images of test panels containing basecoats and clearcoats with and without CAB. The analysis revealed a significant improvement in the orientation of pigments and flop index in the basecoat and clearcoat containing CAB. The optimized pigment orientation and flop index may also lead to reduced pigment costs in formulations, achieving the same metallic effects with less pigment.
When topcoats are applied to basecoats, there is always a risk of redissolution of the basecoat by the topcoat, negatively impacting the appearance, namely due to the disorientation of basecoat pigments. CABs provide redissolve resistance by preventing solvent migration from the topcoat into the basecoat. Figure 6 shows SEM images that were taken at the interface of topcoat and basecoat samples, where one topcoat and basecoat sample contained CABs and one did not. The interface of the sample without CAB appears markedly more diffuse and has a more disrupted pigment alignment due to the solvent migrating from the topcoat into the basecoat.

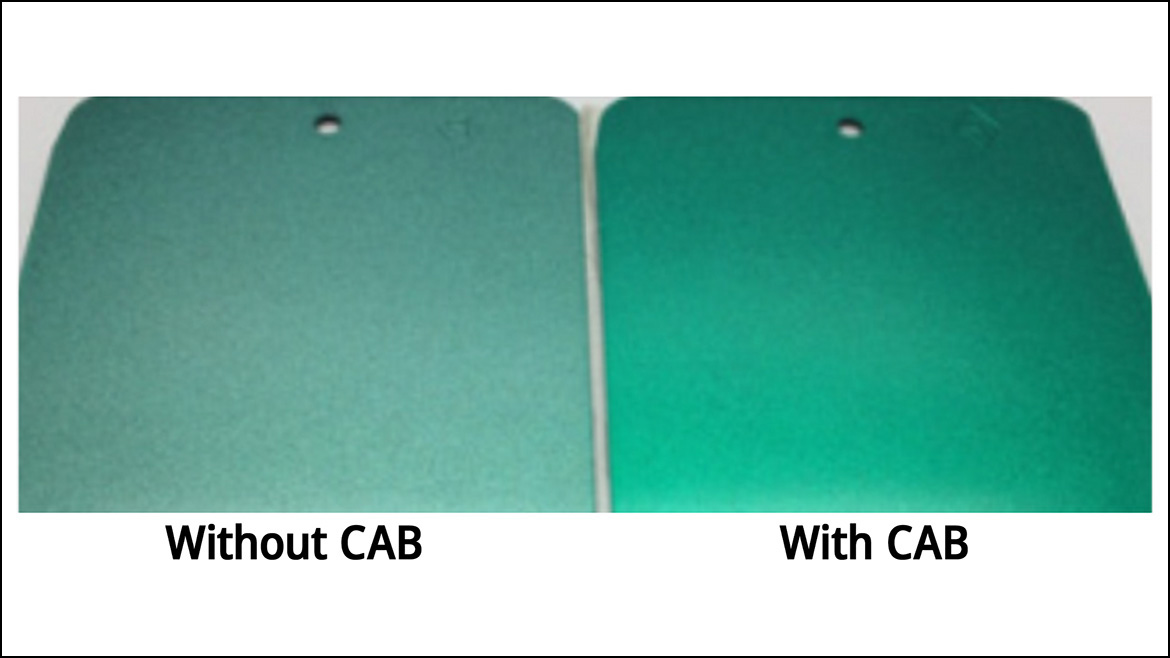
Macroscopically, the impact that CAB provides in terms of redissolve resistance can be more clearly seen in Figure 7. Here, test panels were coated with a metallic-green basecoat and a topcoat prepared with and without CAB. The basecoat and topcoat without CAB exhibited poor color consistency and color development, as well as suboptimal flake orientation due to the dilution and disorientation of pigments in the basecoat.
Better Sustainability
As mentioned earlier in this article, formulations have evolved to favor those promoting higher sustainability, with more water-based and low-VOC solvent-based systems being created. Certain CABs have been developed for compatibility in such formulations to provide performance while aligning with sustainability goals. For example, Solus™ 2100, a low-molecular-weight CAB manufactured by Eastman, is completely soluble in Oxsol 100, a solvent defined as VOC-exempt by the US EPA. Solus™ 3050, another CAB manufactured by Eastman, can be dissolved in blends of organic solvents and water. Using CABs also helps formulators decrease dependence on petroleum-derived raw materials. Some CABs manufactured by Eastman contain 50% bio-content or more.
Summary
Modern automotive refinish coatings must be formulated to minimize downtime during curing, maximize visual quality, and meet sustainability requirements. When cellulose acetate butyrates are formulated into automotive basecoat and topcoat formulations, they significantly reduce dry-to-touch times and improve leveling, pigment orientation and resistance to basecoat redissolution. Certain CABs are also manufactured with high levels of biocontent, and provide compatibility with water-based and low-VOC systems.
ChemPoint is a distributor of Eastman cellulose acetate butyrate, including low-molecular-weight CABs, such as Solus™ 2100 and Solus™ 3050. For more information, visit https://www.chempoint.com.
Solus™ is a trademark of Eastman Chemical Company.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






