Substituted Styrenic Monomers
August 5, 2024
Substituted Styrenic Monomers
August 5, 2024anusorn nakdee, Creatas Video+, via Getty Images.
Styrene (ST) is a common co-monomer used to prepare emulsions that are widely used in architectural coatings, industrial coatings, sealants, and adhesives. Lesser-known aromatic monomers provide considerable advantages and improved performance due to higher boiling points, hydrophobicity, and higher glass transition temperatures. Substituted styrenic monomers have been known for several decades. Certain properties make them very useful in a wide variety of applications. In this article, we compared the performance of polymer emulsions made with substituted styrene, i.e. vinyltoluene (VT), p-methylstyrene (PMS) and 4-t-butylstyrene (TBS), and conventional styrene in direct-to-metal, wood coatings, concrete sealer, and FR/intumescent coating applications. The results demonstrate improved hardness and hardness development, water resistance (whitening, blushing), and UV-resistance differences when TBS is utilized.
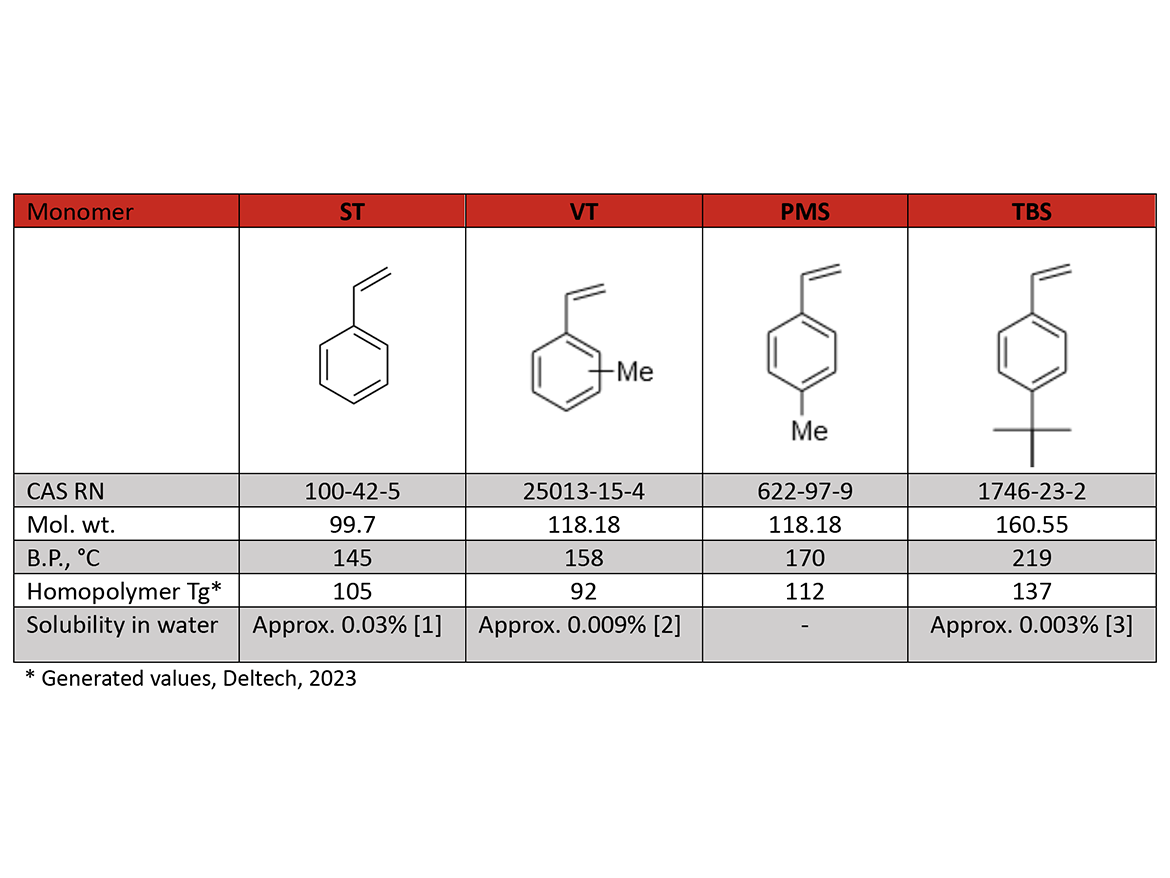
Monomer Data
Vinyltoluene (VT) (55% Meta / 45% Para) Higher boiling point/lower vapor pressure—beneficial in applications where a reactive diluent is used: unsaturated polyesters, alkyds, UV cure, etc. (as a less-hazardous and less-regulated substitute for ST), also in niche applications like plastic scintillators.
Para-Methylstyrene (PMS) (5% Meta / 95% Para) Higher Tg than VT and ST, substitution of PMS for ST can be made with minimal reformulation to improve performance—hardness, rigidity, and thermoresistance when used in specialty coatings, sealants, and adhesives.
Para-Tertiary Butylstyrene (TBS) (5% Meta / 95% Para) TBS is an excellent reactive diluent for unsaturated polyester resin applications such as SMC, BMC, lay-up, spray-up, casting and injection molding, alkyd, and drying oil resins when greater aliphatic compatibility is desired with nonpolar (aliphatic) solvents, multi-block co-polymers in semiconductor manufacturing, rigidity and hydrophobicity in syntactic foam, anti-microbial coatings, membranes for heavy metal separation, fuel cells, etc. TBS imparts higher hardness and weathering resistance to emulsion polymers, enhancing properties of waterborne coatings and adhesives.
Known Applications and Applications in Development
Recently, in addition to the well-established niche applications like plastic scintillators, new applications emerged, making use of the unique chemistry of substituted styrenic monomers. They replace styrene in thermosets and their composites as reactive diluents (RD) for alkyd resins, vinyl esters, and unsaturated polyesters. Styrene, a traditional RD, is a toxic VOC and is listed as a potential carcinogen by NIH.6 TBS and VT are specifically claimed as “high-Tg-hydrophobic monomers," useful in preparation of aqueous dispersions of multistage opaque polymer particles for reduction of TiO2 load.7 They find use in radiation-curable coatings, ink-jet printing ink,11 heavy-metal-separation membranes, anti-microbial surfaces, fuel cells, desalination membranes12,13 for water transport, filtration and separation, and in a solid-yet-transparent material that can provide long-lasting anti-microbial protection on public surfaces.
Other applications include syntactic foams for deep-sea buoyancy applications,24 heat-resistant optical film for polarizing plate,14 high-volume cost-effective manufacturing at the nanoscale (semi-conductors), microlithography, conformance coatings in microchip manufacturing, thin film patterning in semiconductor devices manufacturing and computer memory (spontaneous self-assembly into sub-50 nm domains).15,19 Some other applications include medical devices (blood compatibility),16 multi-block co-polymers with controlled sulfonation for use in ion-exchange resins, polyelectrolytes, oil-water separation membranes,17 elastomeric graft co-polymers with high utility temperature,18 plastic scintillators, where PVT provide higher light output than PST, PMMA,20 solid electrolytes for batteries,21 specialty high-performance coatings—improving protective and barrier properties, thermoresistant plastics for biotech/pharma, medical applications. PMS and, especially TBS, are expected to improve crazing resistance and allow for steam sterilization under mild conditions, which makes them suitable for substantial market for medical devices and biotech /pharma consumables—drug delivery, storage. Yet another application where PMS provides improvement is a co-polymer composition for a polymeric binder in an intumescent coating.22
Glass Transition Temperature (Tg) of Homopolymers
Tg is an important characteristic of an amorphous polymer. Tg of a co-polymer translates into performance characteristics of the end product—hardness, film forming, mechanical strength, thermal resistance, etc. Different applications require polymers with different Tg.
There was a significant discrepancy in Tg values for substituted styrenic monomers published in the literature4,5 resulting in disagreement between literature data and Deltech data (internal communications).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!
Glass transitions for homopolymers of meta- and para-methylstyrene isomers (components of VT) vary significantly: from ~ 75 °C to ~ 107 °C, meta-isomer produces polymer with Tg lower than PST. For the pilot batches of homo VT and PMS, the Tg values were 92 °C and 102 °C, correspondingly. For consistency in Tg values, the monomers were polymerized in vials and Tg was measured by DSC (reported as a midpoint of the transition).
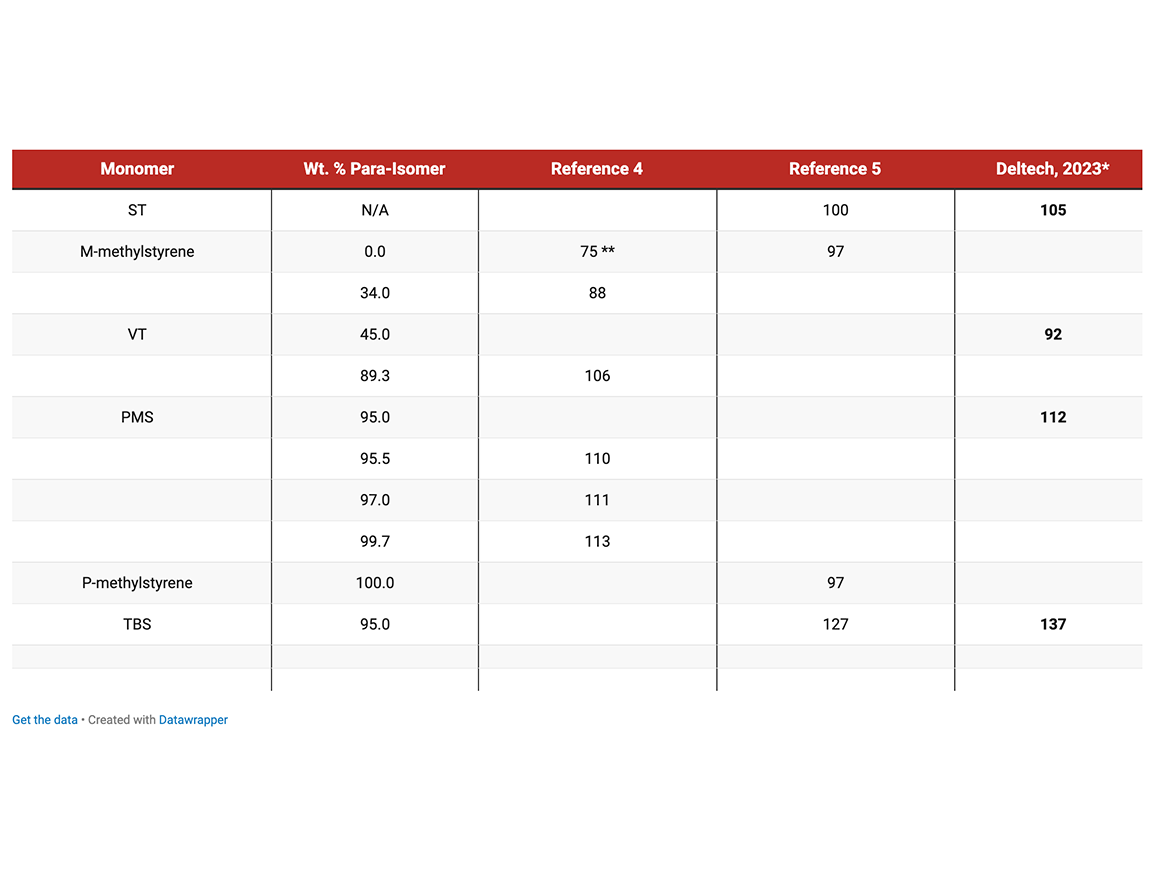
* - DSC, midpoint
** - extrapolated
The glass transition temperature (Tg) of homopolymers has been experimentally measured by DSC and agrees with prior Deltech data.
Experimental
In this study, the emulsions, typical for a few different applications, were evaluated to demonstrate the advantages of using substituted styrenic monomers: direct-to-metal coating (DTM), wood coating (WP), sealant (caulk), concrete sealer (CS) and intumescent coating (FR).
The monomer composition in each series was adjusted to similar Tg with different monomers. Glass transition temperature was in the expected range, with VT giving lower than expected value (the deviation between PMS and VT is attributed to isomer distribution in VT).
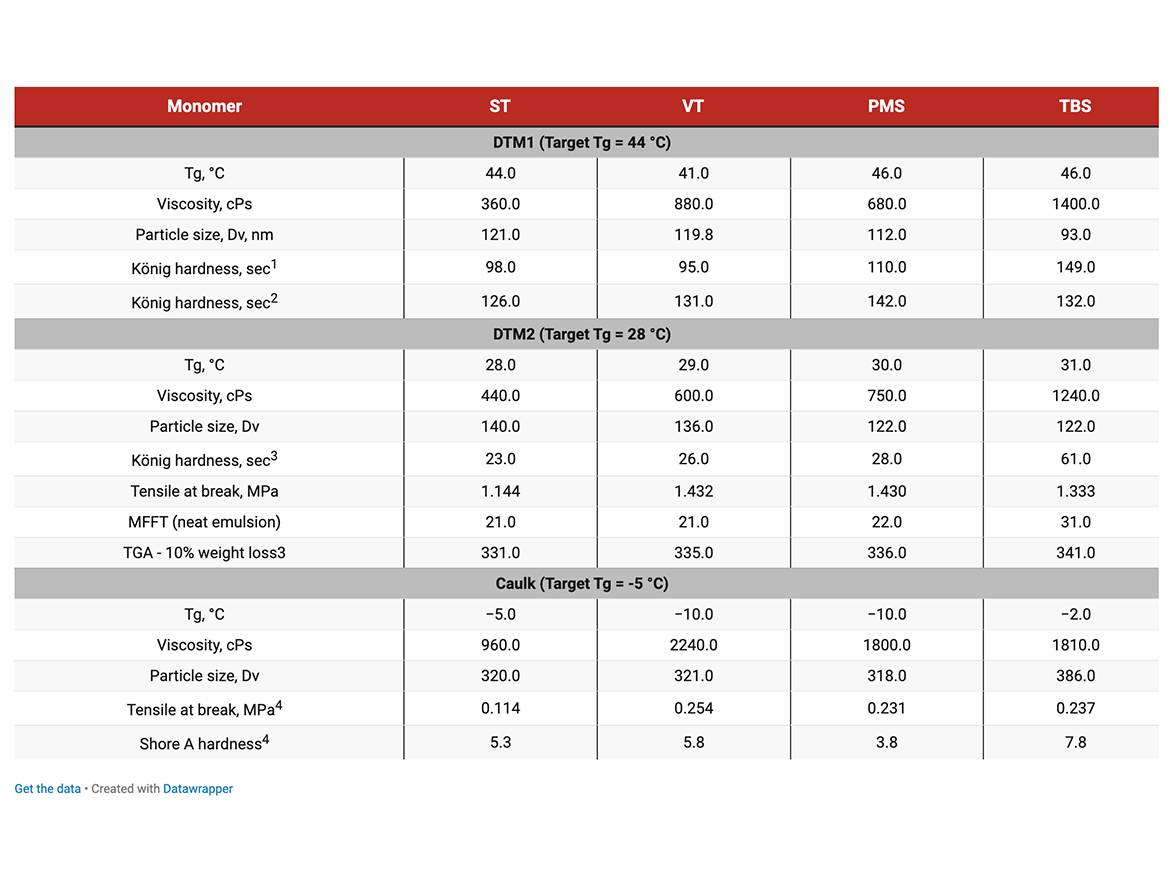
Two sets of DTM emulsions with different target Tg were synthesized and evaluated for hardness, UV resistance, water absorption, and moisture resistance.
A set of low-Tg emulsions was synthesized and evaluated in a single-component sealant (caulk) application per ASTM C920 specification for Type S sealants.
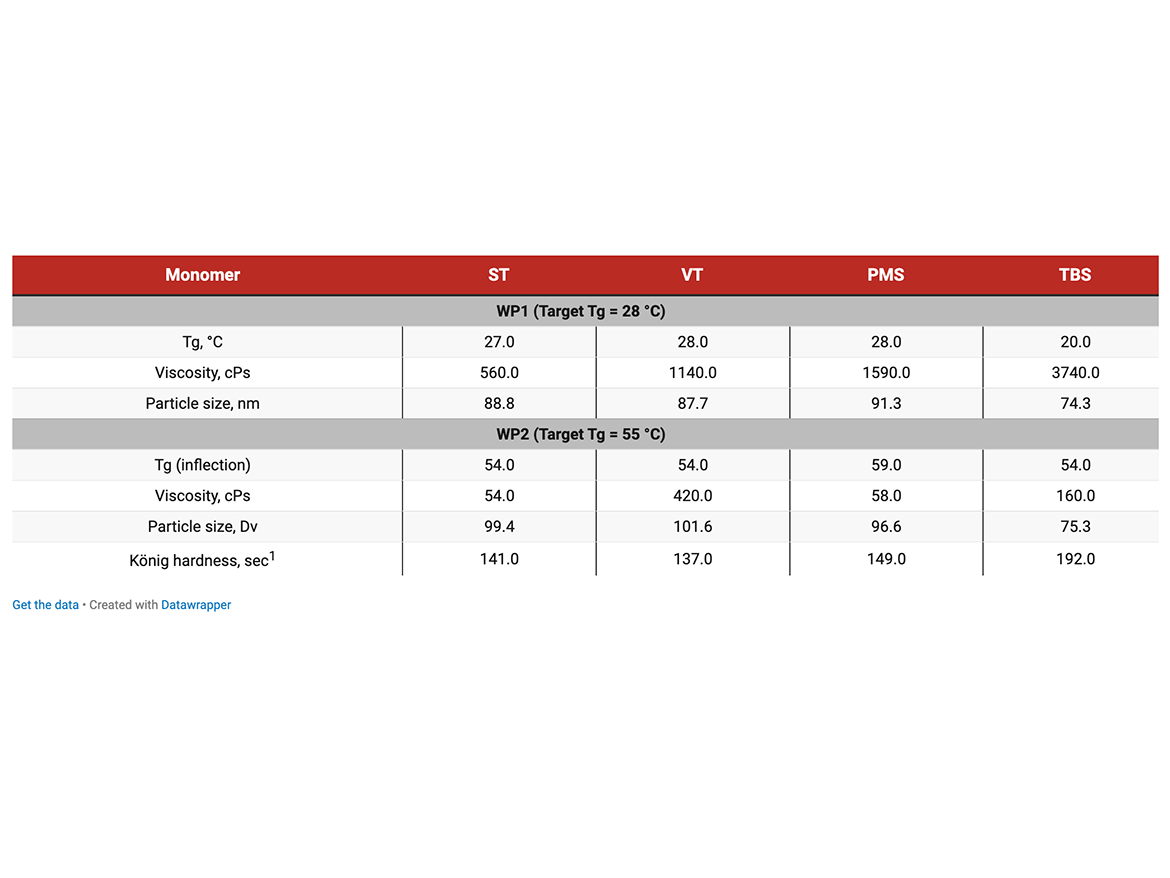
Two sets of polymers with different target Tg were synthesized and evaluated in a wood coating/primer application: WP1 and WP2. WP and WP2 clear coatings were evaluated for UV and moisture resistance. TBS-based emulsions in wood primer applications have demonstrated improved performance consistent with the better moisture resistance and hardness.
1Clear coating on glass, 30% Dowanol DPnB co-solvent, drawdown bar applicator, 3 mil wet
2White gloss DTM paint formula, 3 mil wet (~1 mil dry) on steel
3White gloss DTM paint formula, 5 mil dry film thickness on steel panel
4Formulated caulk
Two hydrophobic co-polymer emulsions with TBS and reduced levels of carboxyl functionality were synthesized and evaluated in concrete sealer and intumescent coating applications.
Test Methods
- Glass transition temperature (Tg) by DSC (ASTM D7426, Standard Test Method for Assignment of the DSC Procedure for Determining Tg of a Polymer or an Elastomeric Compound (Withdrawn 2022)
- Hardness and hardness development (ASTM D4366 Hardness of Organic Coatings by Pendulum Damping Tests (Method A)
- Moisture resistance/hydrophobicity
- Water soak (ASTM D471, Standard Test Method for Rubber Property—Effect of Liquids, or ASTM D 570, Standard Test Method for Water Absorption of Plastics)
- Contact angle (visual observation)
- Water spot test (visual observation)
- Blush test23
- UV resistance (ASTM G154, Standard Practice for Operating Fluorescent Ultraviolet (UV) Lamp Apparatus for Exposure of Nonmetallic Materials)
- Minimum film forming temperature (ASTM D2354, Standard Test Method for Minimum Film Formation Temperature (MFFT) of Emulsion Vehicles)
- TGA (ASTM E1131, Standard Test Method for Compositional Analysis by Thermogravimetry)
- Bunsen burner test
- Chemical resistance (ASTM D1308, Standard Test Method for Effect of Household Chemicals on Clear and Pigmented Coating Systems)
- Latex viscosity (Brookfield LV viscometer, spindle #4 @ 20 rpm)
- Latex particle size (laser diffraction, Microtrac Wave)
Results
Hardness and Hardness Development Glass transition temperature is an intrinsic property of a polymer that defines important performance characteristics like film formation properties and hardness. Depending on co-polymer composition, the properties can be further improved even at the same Tg.
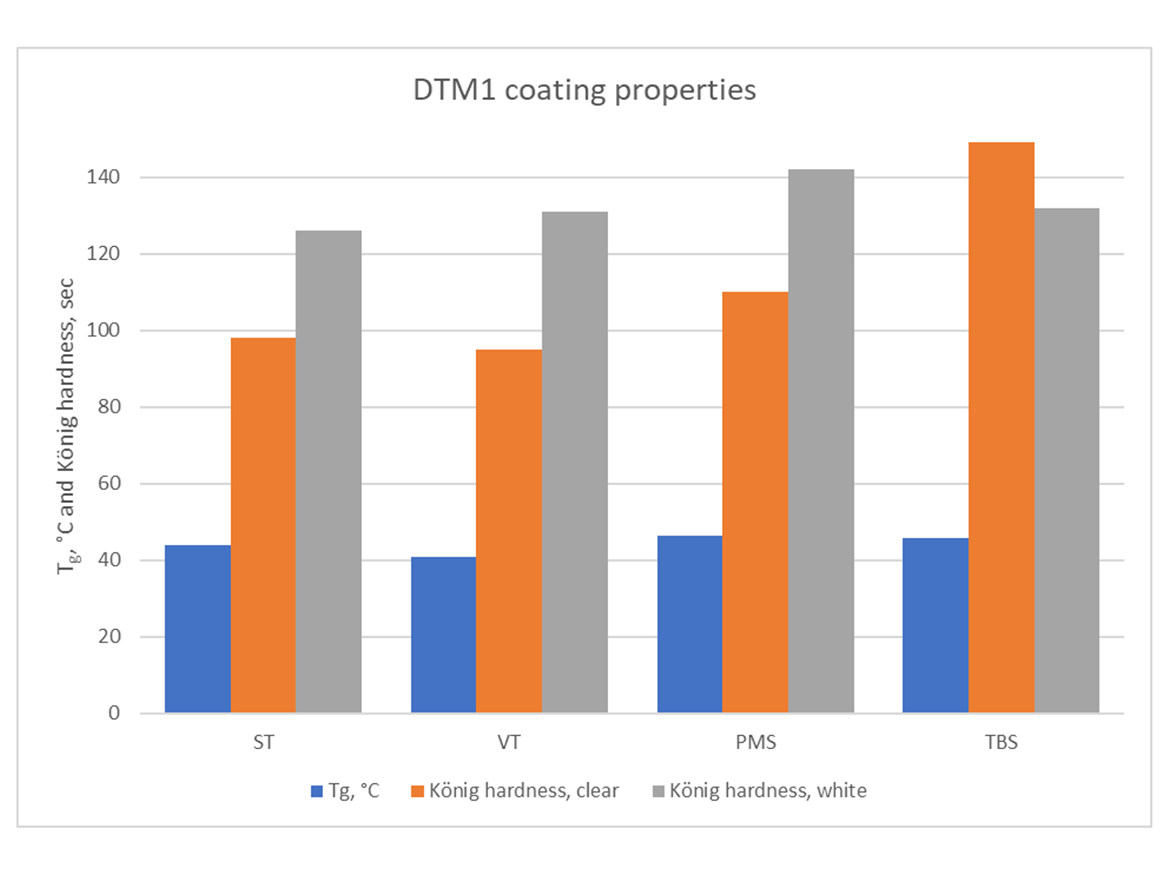
Clear coating: 30% Dowanol DPnB cosolvent, drawdown bar applicator, 3-mil wet on glass panel. White gloss DTM paint formula: drawdown bar applicator, 3 mil wet on steel panel.
Co-polymer emulsions with PMS and TBS produced films with higher hardness. In case of TBS, hardness at similar Tg is probably related to a different co-polymer and particle morphology. It deserves further investigation. The Tg difference between PMS and VT could be attributed to isomer distribution in VT, and, possibly, to the co-polymer and particles morphology.
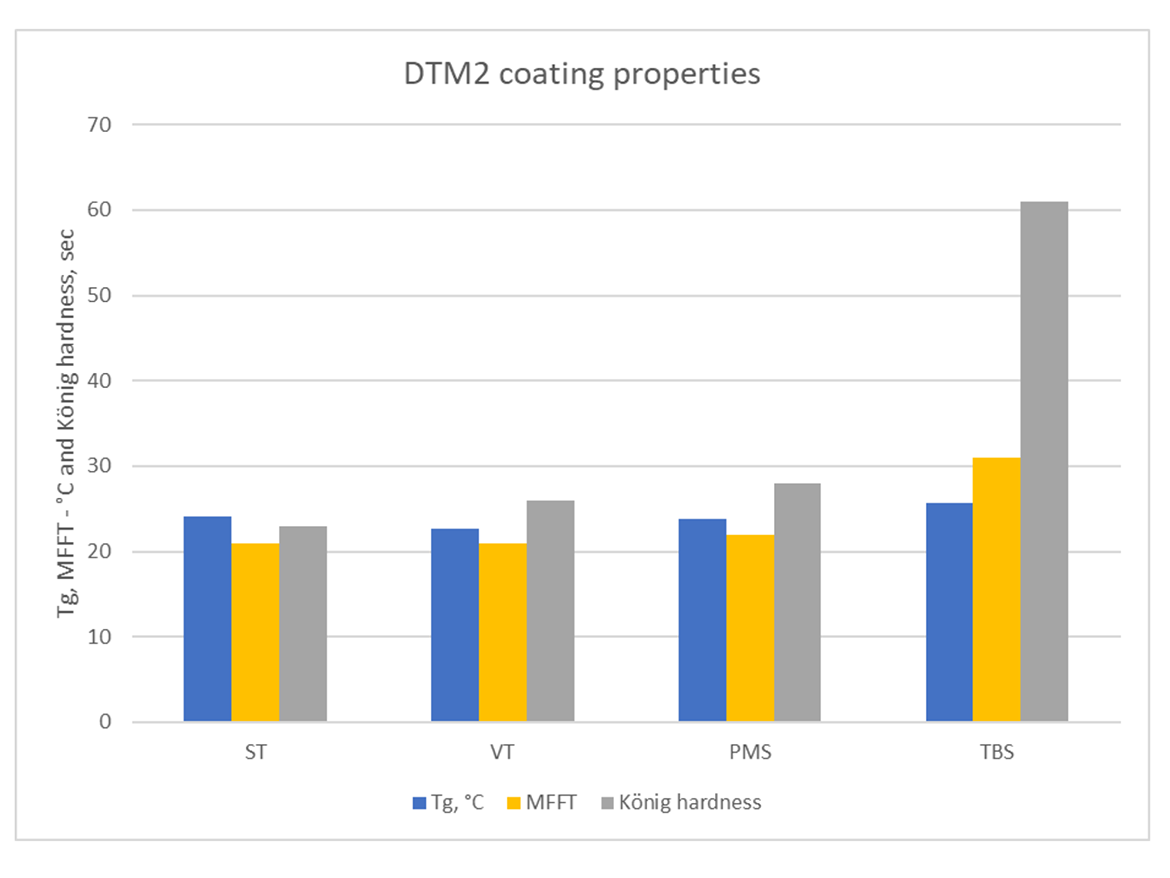
White gloss DTM2 paint formula, coating hardness is measured on 5-mil dry film thickness on steel panel.
Hardness with TBS was notably higher. MFFT with TBS was higher compared to the observed Tg, which may require some adjustments for coalescing aid in paint formulations.
One of the important properties of paints is drying time—this was evaluated by hardness development test. Results are shown in Figure 3.
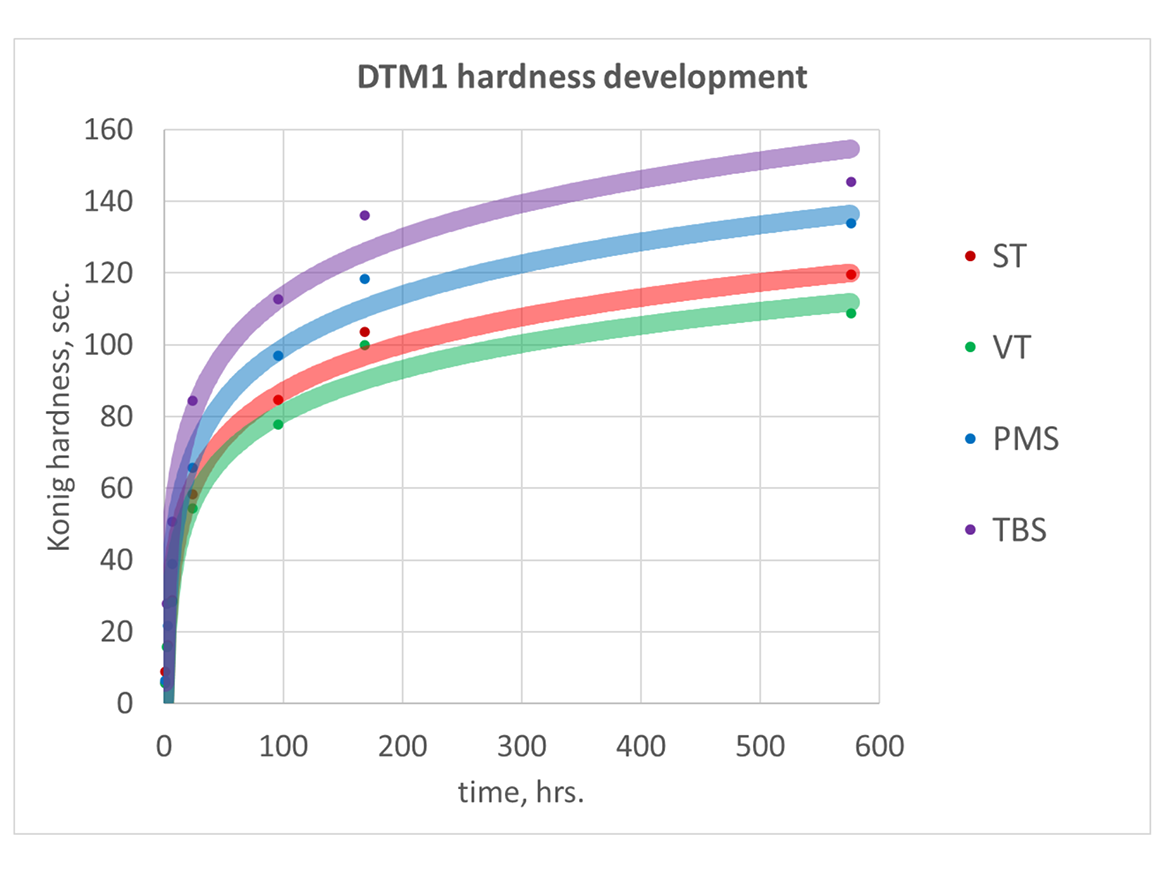
Clear coating with butyl cellosolve @ 25%, as coalescing aid, drawdown 3-mil wet on glass.
PMS and, especially TBS, provided notably better performance—much higher hardness and faster hardness development at similar Tgs. VT produced softer coating, and hardness development was slower than ST. The absence of correlation with Tg is most likely related to differences in film forming properties due to co-polymer composition and specific particle morphology resulting from monomer reactivity differences and deserves more thorough examination.
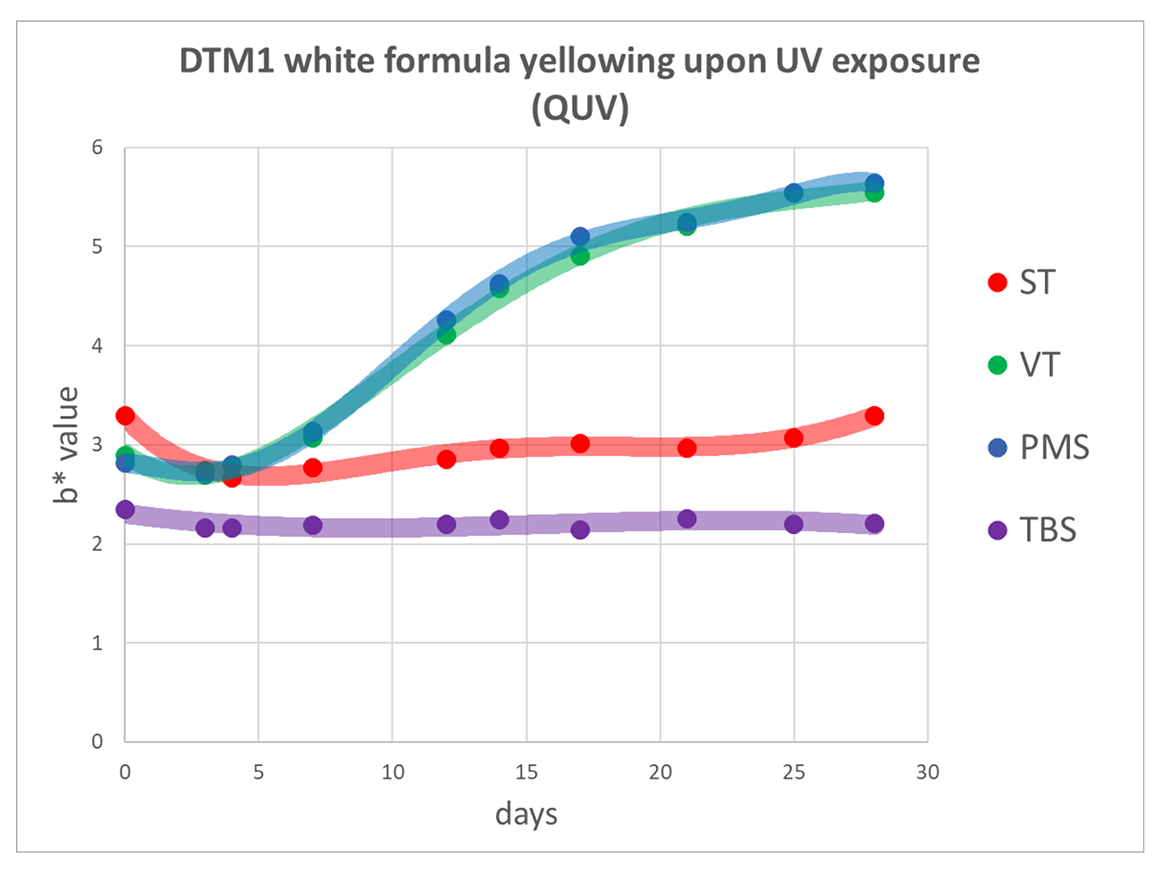
UV Resistance—Differences in Yellowing of Polymers Made with Substituted Styrene Monomers UV resistance of the polymers in clear and formulated white coatings was evaluated using QUV method by measuring b* value of clear and white gloss coating formulation (blue-yellow coordinate in CIELAB color space) after exposure to UV (Figures 4, 5, 6 and 7).

White gloss DTM paint formula, 3-mil wet drawdowns on standard Leneta cards (DTM1 series).
White gloss paint formula, 3-mil wet drawdown on standard Leneta card.
WP clear coatings were evaluated for UV resistance using QUV in comparison to two competitive all-acrylic products (>500 hrs.) (Figures 5, 6).

Neat emulsions with 30% (w/w polymer) cosolvent DPnB, drawdowns 3-mil wet on standard Leneta cards. From left to right: ST, VT, PMS, TBS, TBS (2), Acrylic 1, Acrylic 2.

Neat emulsions with 30% (w/w polymer) cosolvent DPnB, drawdowns 3-mil wet on standard Leneta cards.
TBS-based polymer demonstrated UV resistance comparable to all-acrylic polymers, known for good UV resistance. VT and PMS-containing polymers showed more pronounced yellowing in both, white gloss DTM1 formula, and WP clear coatings.
TBS showed excellent resistance to UV light—in photooxidation reactions, stabilization effect by steric hindrance of the bulky t-butyl group overcomes the effect of electron density donation. TBS co-polymer demonstrated robust resistance to UV exposure comparable to all-acrylic polymers. VT and PMS discolored more than ST because of electron density donation by alkyl substituents on the aromatic ring.
Water Resistance—Water Spot Test DTM1 and WP2 clear coatings were evaluated for water resistance using a spot test in comparison with two competitive all-acrylic products.

Top: drop of water on the coating. Bottom: water drop removed. From left to right: ST, VT, PMS, TBS, Acrylic 1, Acrylic 2.
The same trend was observed with two types of co-polymers —DTM1 and WP2—TBS was the most resistant. The all-acrylic coatings swelled in water and wrinkled. Acrylic 1 did not fully recover after drying.
Spot test demonstrated improved water resistance when Deltech monomers VT, PMS, and TBS are used. The test showed a clear advantage of TBS monomer.
Water Resistance—Blush Test Blush resistance is another test for water sensitivity and is more sensitive than the water absorption test. In this study, we used a modified blush test by immersion in water with instrumental measuring of whitening degree by using a colorimeter for graphical representation of the data.23 Veocryl emulsion was used for comparison (co-polymer containing VeoVa 10 hydrophobic monomer). Clear coatings were cast on a glass plate at 3-mil wet thickness and dried for 7 days at RT.
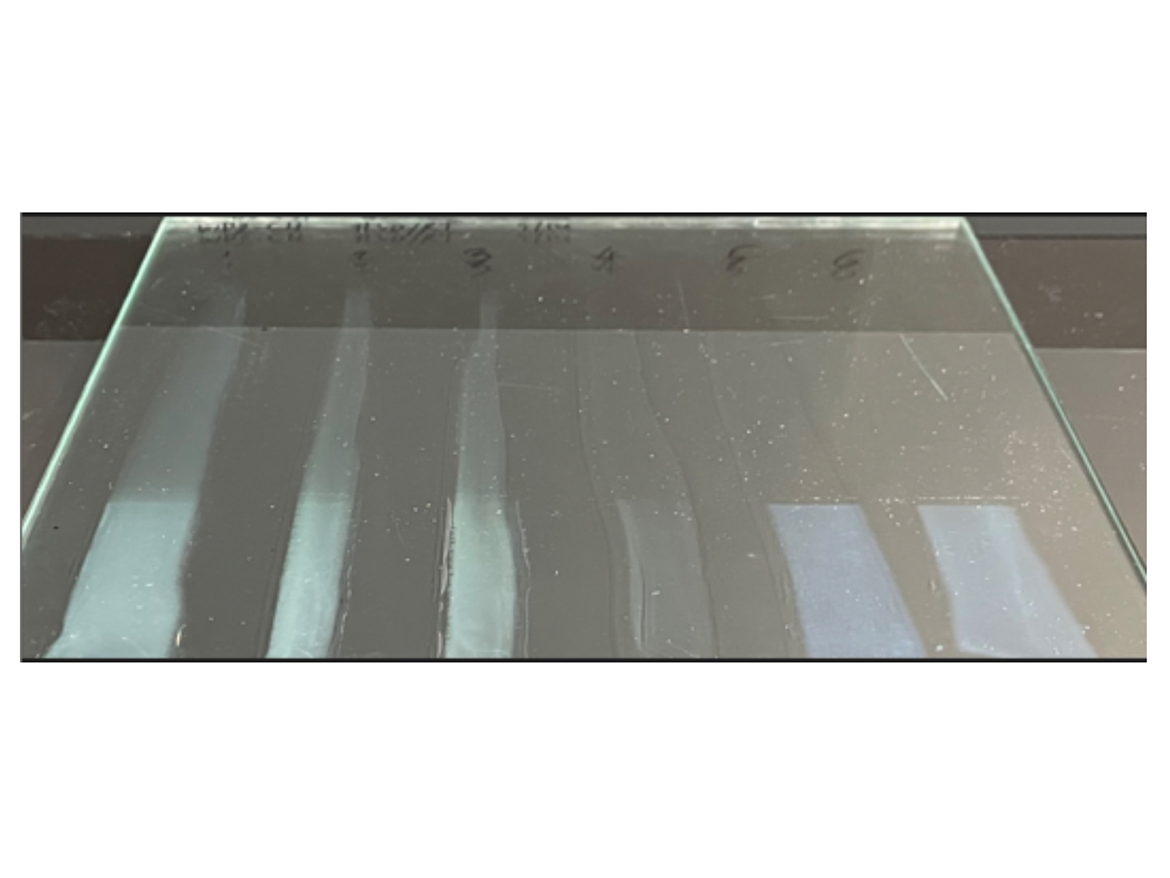
From left to right: ST, VT, PMS, TBS, Acrylic 1, Acrylic 2.
TBS is clearly the most resistant (#4). Acrylics (#5, #6) were covered with blisters (not immediately visible on the photo).
To quantify the visually observed blushing, loss of film clarity was determined by measuring the color change (whitening) of clear films cast on glass after immersion in water at room temperature. The whitening was recorded as CIELAB L*value change over time. Measurements were done using BYK color guide colorimeter.
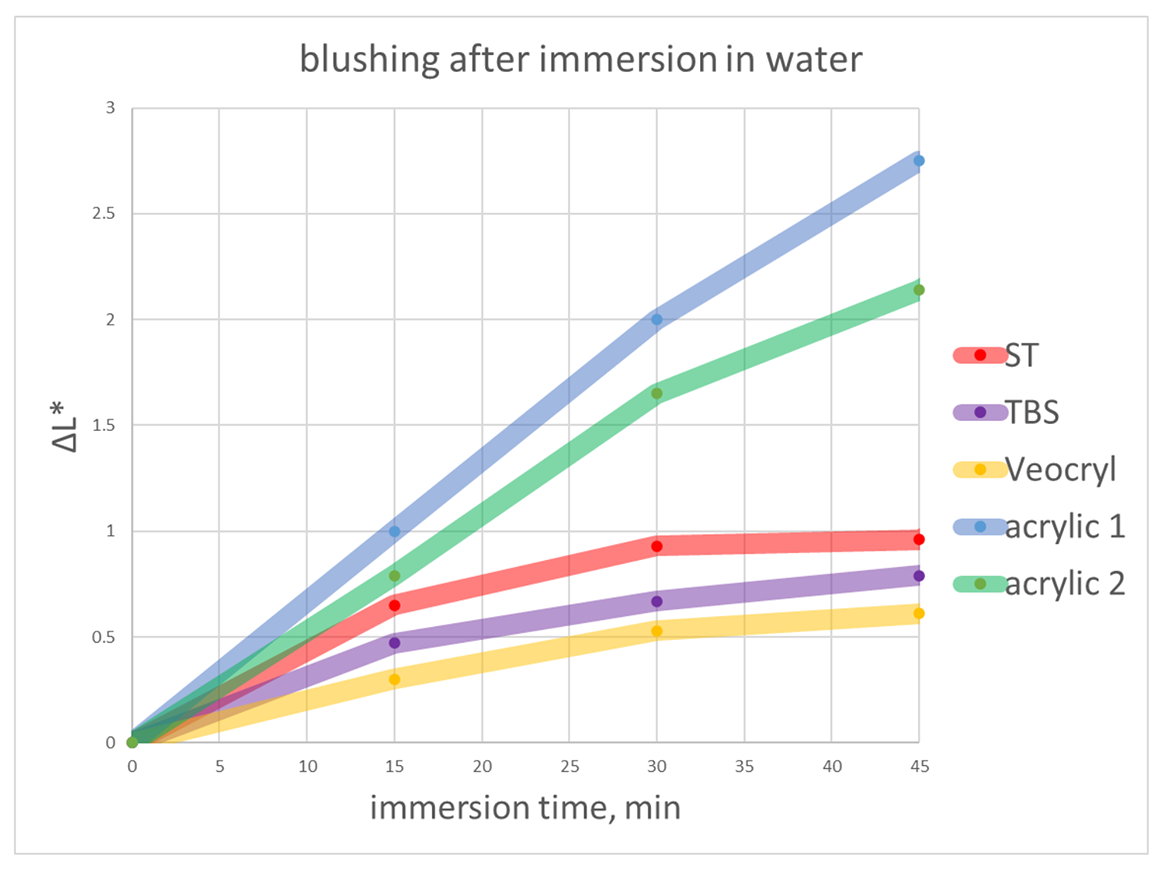
Co-polymer with TBS is more resistant than ST and is similar to self-crosslinking VeoCryl co-polymer in this test. Acrylic coatings blushed notably more and were covered with blisters after immersion in water.
Hydrophobicity by Water Soak (ASTM D570, ASTM D471) and Water Spot (Initial Contact Angle) Water absorption is intimately related to mechanical strength, appearance, and dimensions. It is a direct measure of polymer hydrophobicity. The most universal and easiest method is the water absorption test by immersion (ASTM D570). To perform the test, specimens in the form of free films of sufficient thickness (~1 mm), emulsions formulated with co-solvents were cast in polyethylene molds and dried at room temperature with subsequent annealing at 250 °F on a non-stick plate. Water absorption was measured as weight change after immersion in water for 24 hours.
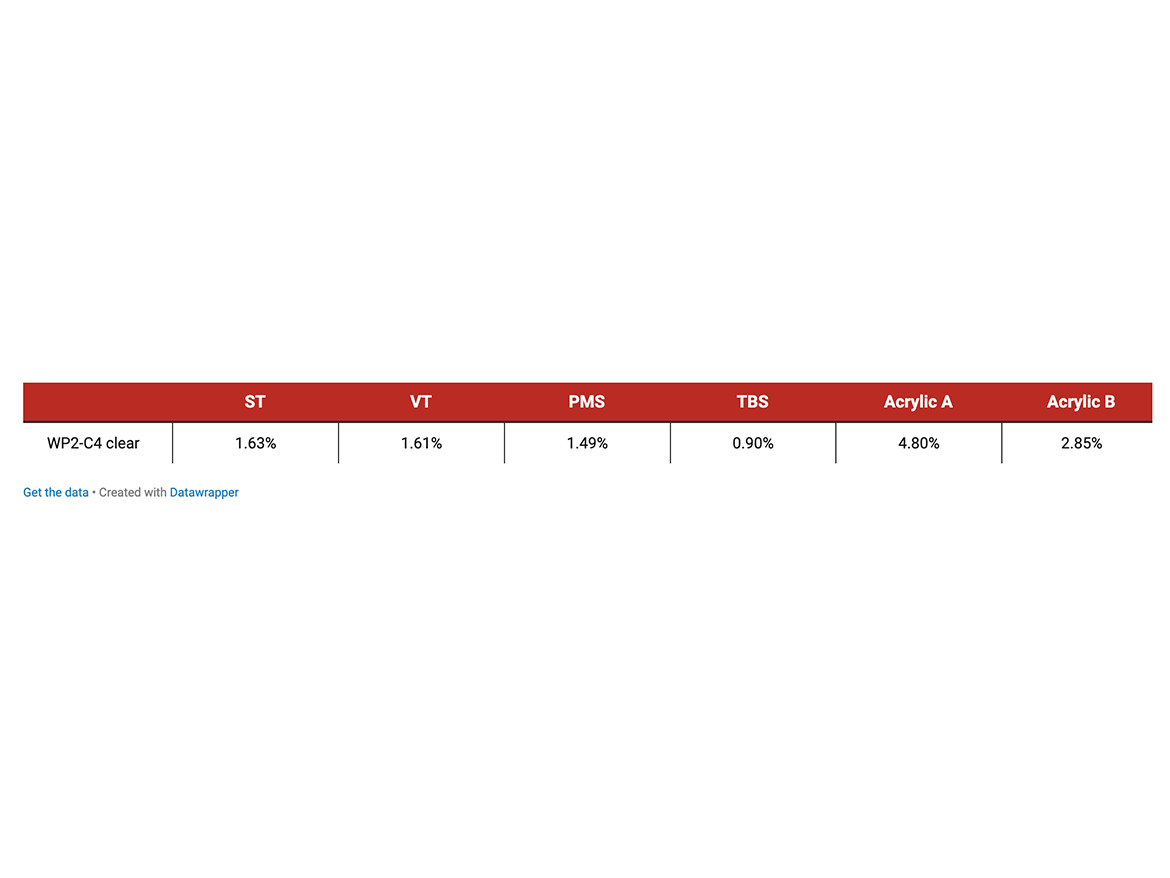
Water soak (ASTM D471) is another standard test for water absorption. It was used to measure moisture sensitivity of white gloss paint and caulk formulations based on corresponding DTM2 and caulk polymer emulsions made with different styrenic co-monomers. Films were cast 40-mil wet on Leneta Form RP-1K release paper, dried at room temperature for 1 week, removed from the release paper and overturned, then dried for one more week. A pre-weighed film sample measuring 1” x 2” was immersed in water at 23 °C for 1 week. The sample was removed, briefly dipped in acetone, blotted dry with a lint-free towel, and re-weighed.
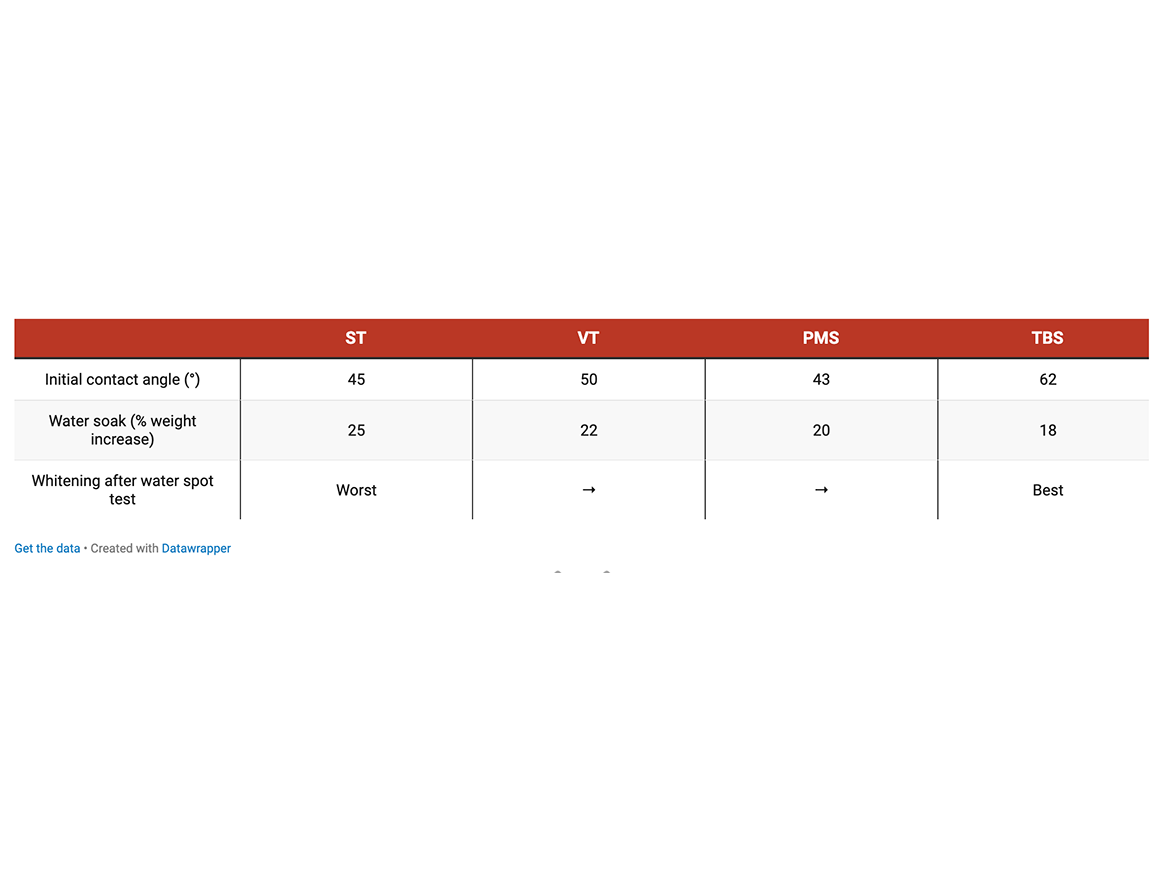
Another method frequently used to ascertain hydrophobicity of a material surface is the contact angle measurement. The initial contact angle was measured by observing a drop of water placed on the coating surface. Films were cast on Leneta 7B chart at 5-mil wet film thickness and dried at room temperature for 2 weeks. A large drop of water was placed on each film and left to sit undisturbed for 6 hours. The initial contact angle was observed and the color change of the latex under the water drop was visually observed at the end of the test.
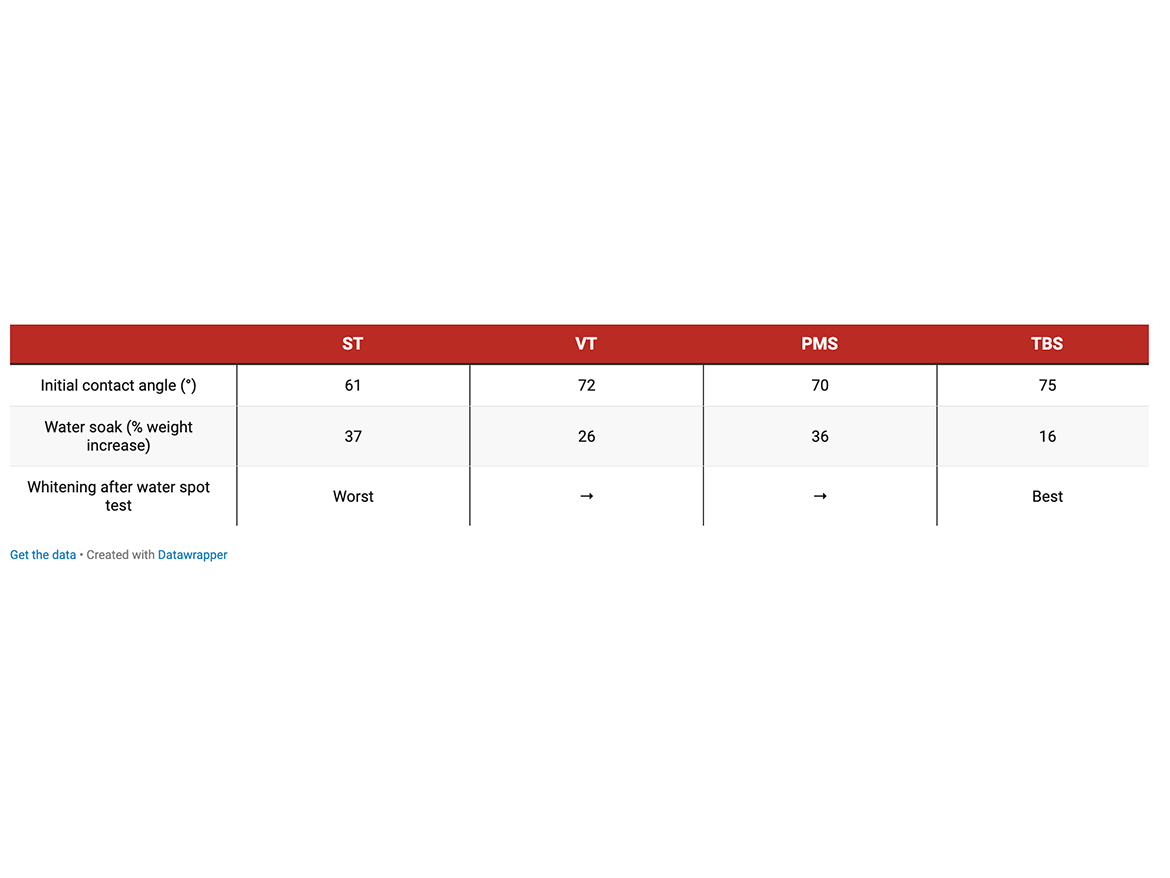
The water absorption/water soak tests and contact angle measurements clearly show that substituted styrenic monomers exceptionally increased hydrophobicity of both white DTM paint and single-component caulk materials. TBS produced the highest degree of hydrophobicity increase. Tables 6, 7, and 8 show the lowest water soak/absorption and the highest contact angle for the co-polymer with TBS and significantly higher absorption for all-acrylic competitive products.
VT and PMS are expected to have similar solubility in water. To maintain Tg of acrylic/styrenic co-polymer, more VT than PMS is needed, and this, probably, explains somewhat better water soak test results for VT-based caulk formula.
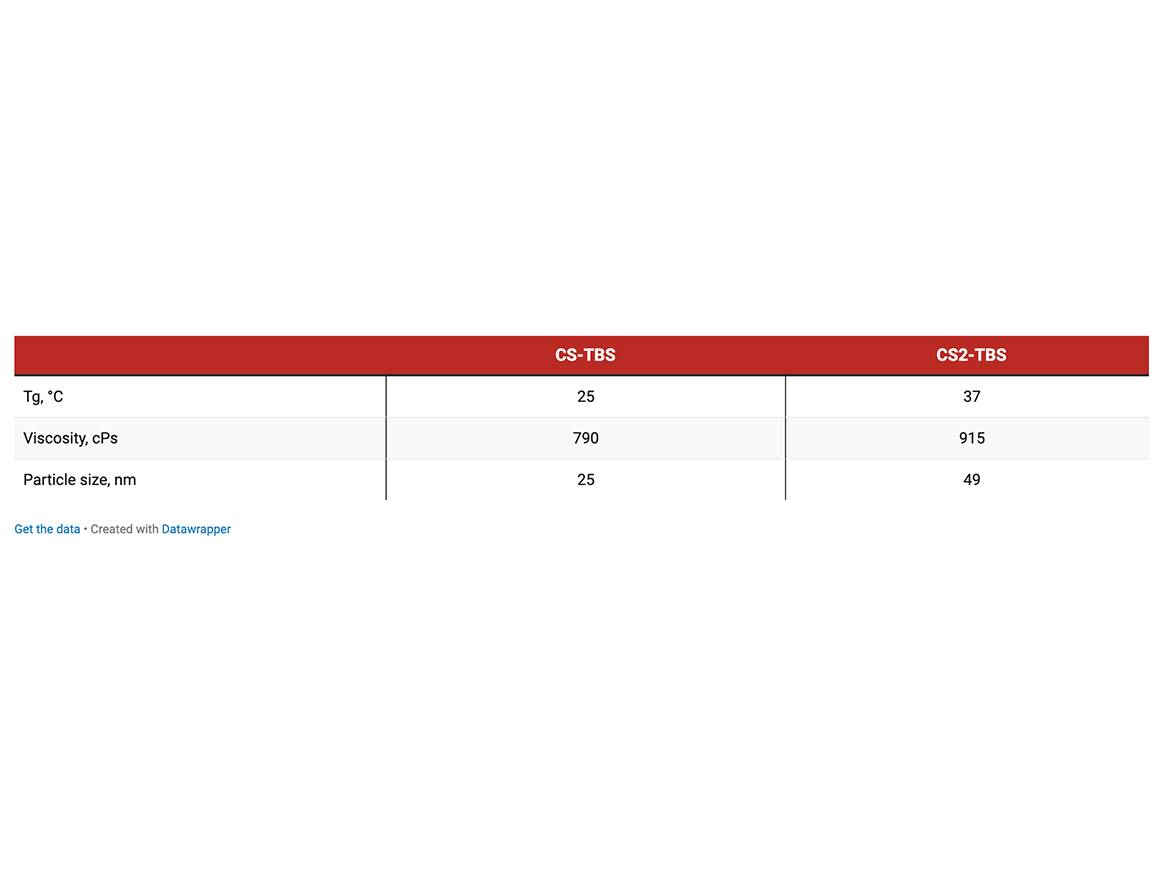
Concrete Sealer Application (CS) CS (non-crosslinking) and CS2 (self-crosslinking) emulsions containing TBS were evaluated in concrete sealing application against commercial product according to ASTM D1308 Effect of Household Chemicals (covered spot method).
The test concrete specimens were coated using brush by weight, to provide approximately 3 mil dry coating thickness. Three drops of each chemical were applied on a coated concrete surface, covered with a watch glass and left at ambient conditions for 24 hours. After exposure, the liquid was wiped off by tissue and the spots were examined and rated as follows: 4—untouched; 3—reduction in gloss; 2—softening, swirl, or blistering; 1—complete failure.
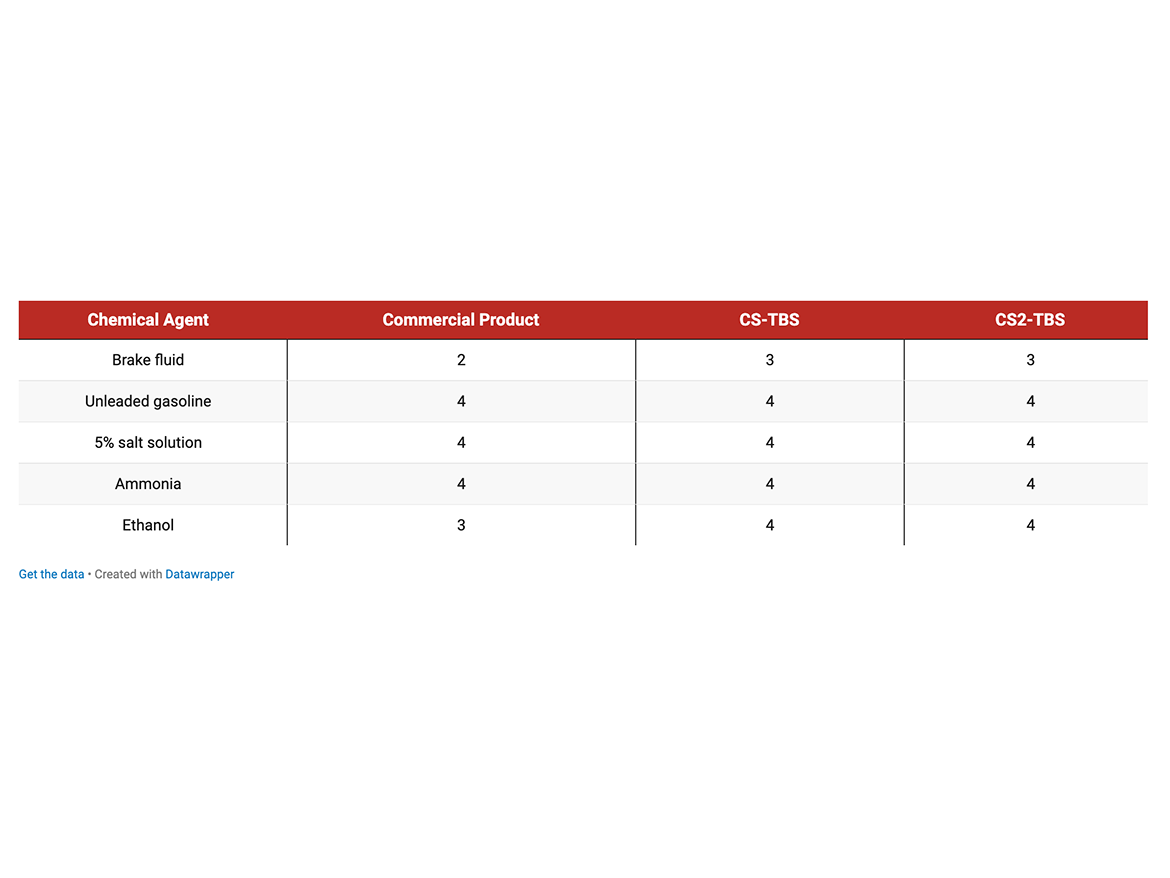
The co-polymer emulsions containing TBS improved resistance to brake fluid and ethanol. TBS matched commercial performance in exposure to salt solution, gasoline, and ammonia.
FR/Intumescent Coating U.S. Patent No. 7,417,09122 specifically claims the benefits of using PMS and TBS in polymer binder for intumescent coatings in combination with crosslinking (reticulated polymer). Two CS emulsions containing TBS were evaluated in intumescent coating application by Bunsen burner test.
Aluminum 4×6” panels were coated at 15-mil wet thickness, dried at RT/7 days and tested as follows: the panels were placed at 45° angle, exposed to 0.5-inch Bunsen burner flame for 5 minutes. Flame spread and char formation is evaluated visually.
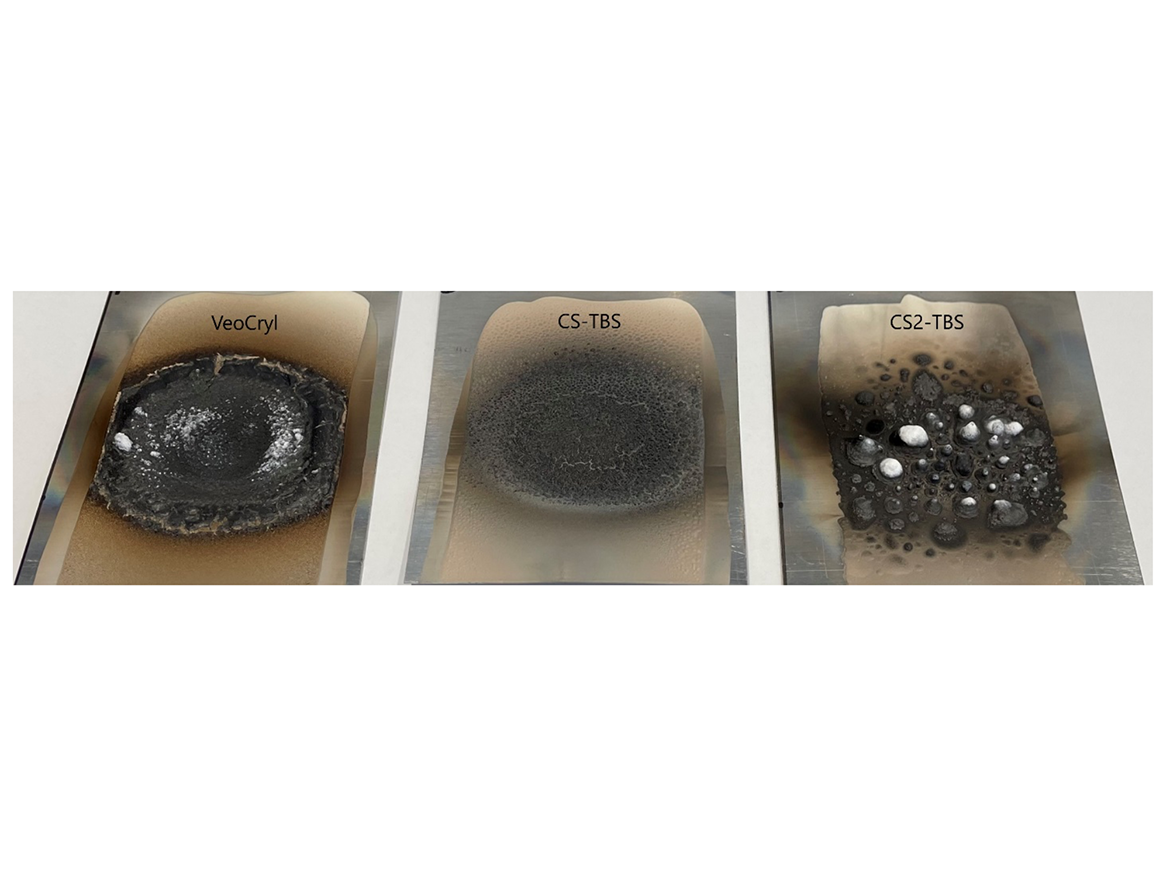
Left to right: commercial VeoCryl emulsion, non-crosslinked CS-TBS emulsion, self-crosslinking CS2-TBS emulsion.
The combination of TBS and crosslinking drastically increases expansion of the coating upon exposure to open flames and is expected to improve thermal insulation properties. It is comparable to the commercial emulsion.
Conclusions
Deltech monomers, VT, PMS, and TBS were evaluated in combination with different acrylic co-monomers for properties adjustment, suitable for DTM, wood coating applications, concrete sealer, and intumescent coatings. Co-polymer emulsions with PMS and, especially, TBS-containing co-polymers with similar glass transition temperatures (Tg) produced superior hardness and hardness development, demonstrated by tests on clear DTM and WP coatings and pigmented paint formulations. TBS-containing co-polymer emulsions demonstrated exceptional UV resistance, on par with all-acrylic products, Emulsions based on TBS exhibited exceptional water resistance, as shown by water spot, soak and blushing tests, outperforming all-acrylic self-crosslinking and VeoVa monomer-based polymer emulsions. VT higher boiling point (compared to ST) makes it more acceptable as a reactive diluent from EHS standpoint. PMS has potential in high-end applications. Two TBS-based emulsions, WP-TBS and WP2-TBS, have been evaluated in wood primer application and results of evaluation demonstrated exceptional sandability and good waterborne topcoat hold-out after recoating. WP2 was slightly better for the recoat. TBS-based co-polymer emulsions have been evaluated in concrete sealer and intumescent coating applications and showed adequate performance.
Acknowledgements
I would like to thank Deltech for sponsoring and supporting the research presented in the paper. I also want to thank the group of StanChem Resins employees who helped me with the experimental part, provided the facilities and analytical instruments, and provided invaluable support to plan the experiments and interpret the results: Buce Holt, Ray Rouleau, Eni Cini, Matt Auchy, and many others. Special thanks to 1st Source Research team, who helped in evaluation of DTM and caulk formulations: Gerald Vandezande, Abigail Bridges, Kenneth Price, Wendy Daniels.
*Product and process patents pending.
References
1 Arriaga, V.; Vanaken, D. Branched Vinyl Ester Monomers for Hydrophobic Emulsion Polymers. PCI Mag (2013).
2 Yaws CL et. Al.; Chem. Eng., 97: 177-81 (1990) https://pubchem.ncbi.nlm.nih.gov/compound/3-Methylstyrene#section=Other-Identifiers.
3 Dunn, A.S. Latex Particle Nucleation in Emulsion Polymerization, Eur. Polym. J., 25, 7–8, 1989, 691-694. cited: Dow Chemical Co. Technical Bulletin on tert-butyl styrene monomer.
4 Canterino, P. J. Chemically Cross-Linked Poly(p-methylstyrene), U.S. Patent Number 4230836, 1980 (DSC, onset).
6 Robertson, B. A. Low-HAP and Zero-HAP Polyester Resin Systems and Articles Prepared Using Same. U.S. Patent No. 7723431, 2010.
7 Bardman, J. K. Aqueous Dispersion of Multistage Polymer Particles. U.S. Patent No. 11186741, 2021.
8 Zhang, L, et. al., Water Resistant Polymers for Personal Care. U.S. Patent No. 9820929, 2017.
9 Brennan, D. J., et. al. Compositions Comprising Polyether - Urethane - Urea Additives for Block Resistance and Open Time. U.S. Patent No. 10654964, 2020.
10 Karikari A. S., et al., Epoxy-Fortified Floor Polishes. U.S. Patent No. 9969904, 2018
11 Guenther, G., et. al. Ink-jet printing ink. U.S. Patent No. 9447295, 2016.
12 Willis, C. L., et. al. Sulfonated Block Co-Polymers, Method for Making Same, and Various Uses for Such Block Co-Polymers. U.S. Patent No. 7737224. 2010.
13 Kim, J. H., et. al. Homogeneous Cation-Exchange Composite Membrane U.S. Patent No. 10543463. 2020.
14 Fujino, Y. Optical Film and Polarizing Plate. U.S. Patent No. 10941236, 202.
15 Kim, D. H. Method of Forming Topcoat for Patterning U.S. Patent No. 10755942, 2020.
16 Tsai, B.H; Chuang, Y.H.; Cheng, C.H.; Lin, J.C. Sulfonation and Characterization of Tert-Butyl Styrene/Styrene/Isoprene Co-polymer and Polypropylene Blends for Blood Compatibility Applications, Polymers 2020, 12(6), 1351.
17 Zhao, Y.; Shen, L.; Yuan, Y.; Xiao, L.; Cai, J.; Lu, Z.; Hou, L. Preparation of Porous Poly(4-tert-Butylstyrene) Based Monoliths with High Efficiency for Oil-Water Separation via High Internal Phase Emulsion Template, J. Appl. Polym. Sci. 2023, 140(18), e53801 .
18 Synthesis of Multifunctionalized Graft-Type Polyolefin-Based [polyolefin-graft-poly(t-butylstyrene)] Elastomers with a High Utility Temperature, Macromol. Chem. Phys. 2017, 218, 1700298.
19 Application of Block Polymers to Patterning Thin Films for Use in the Manufacturing of Semiconductor Devices and Computer Memory: e.g. Photopatterning of Block Co-polymer Thin Films. ACS Macro Lett. 2016, 5, 460−465.
20 Hurlbut, C. R.; Kusner, M. R. Organic Scintillators for Modern Calorimetry, 2nd International Conference on Calorimetry in High-energy Physics, 1991, 179—183.
21 Zhenting, L. Vinyltoluene/Acrylamide-Based High-Elasticity Storage Battery Sealant and Manufacturing Method Thereof, CN Application No: 201610486517, 2016.
22 Magnet, S., et. Al., Polymer Binder for Intumescent Coatings U.S. Patent No. 7,417,091. 2008.
23 Bassett, D.R. Hydrophobic Coatings from Emulsion Polymers. Journal of Coatings Technology, 73(1), 43–55, (2001). doi:10.1007/bf02698496.
24 https://www.mica-tron.com/syntactic-foams/.








