Phyllosilicate Clay Thickeners for Latex Paints
Winner of the PCI Award for Technical Excellence at the Waterborne Symposium

Latex paints based on dispersions of acrylic, styrene-acrylic, vinyl-acrylic and other polymers are a composition of ingredients that ensure a number of properties, starting from liquid parameters, such as stability in the packaging, and broadly understood workability related to application, as well asparameters related to drying and forming the coating, and properties after drying, such as scrub resistance, gloss, weatherability, etc. Ensuring a perfect balance of these properties and correlating them with the price of the final paint is not an easy task for the formulator.
One of the most difficult to achieve is the paint's workability, which includes a number of rheological properties responsible for easy application with a brush, roller or spray, but also the elimination of spattering or the ability to apply a thicker layer without dripping. Appropriate rheological properties are also needed to ensure the parameters of the liquid paint during its storage. Gravity and intermolecular forces cause settling, syneresis, viscosity drift, etc. In addition, one should not forget about the need to buffer the viscosity after adding pigment concentrates in the tinting process without changing the application properties of the paint.
The group of paint ingredients responsible for these properties are rheological additives, also known as thickeners. These are agents that are employed to generate thixotropy and/or pseudoplasticity in coating materials. Rheological additives play a particular role in ensuring rheological properties at every stage of the paint's life in the liquid state, from its production and pouring into packaging, through its application and behavior immediately after application to the substrate, to its drying process. Rheological additives are not solely responsible for these properties, as their effectiveness depends on the interaction with other ingredients in the formulation, e.g. dispersants or binders. However, their role is crucial in relation to the type of rheological additives, methods of building the rheological structure and pigment volume concentration (PVC) of the paint.
To obtain all the application properties that are responsible for rheological additives in latex paints, organic thickeners are used, such as cellulose ethers (e.g. HEC—hydroxyethyl cellulose), polysaccharides (e.g. xanthan gum), acrylic thickeners (e.g. HASE—hydrophobically modified alkali-swellable emulsions), or polyurethanes (e.g. HEUR—hydrophobically modified polyether-based polyurethanes), but also inorganic ones, represented by phyllosilicates, also known as layered silicates. Their examples are bentonite, sepiolite, attapulgite, or even smectite. The role of the formulator is to select rheological additives in such a way as to ensure appropriate viscosity during all operations, from paint production, through pouring and packaging, storage to application, including all additional parameters such as leveling, resistance to sagging, spattering, etc. Latex paint formulations usually cannot contain one thickener, as it is rarely effective enough to satisfy all rheological parameters. Therefore, usually two, three, or sometimes four rheological additives build the rheological structure of latex paints. This article discusses the basic characteristics of phyllosilicates thickeners, and provides an introduction to understanding their performance in formulations in order to use them to build the rheological properties of latex paints.
Phyllosilicate Clays
Phyllosilicates are a group of minerals that consist mainly of silica and aluminum, with some oxygen and hydrogen. Their structure is composed of layers in which silicon and aluminum atoms are connected to each other through oxygen atoms, creating flat sheets. These sheets can be connected to each other by metal ions or other elements, creating a layered structure. Examples of phyllosilicates include minerals such as kaolinite, pyrophyllite, talc, and chlorite. They are often found in sedimentary, metamorphic and volcanic rocks, and play an important role in geology, soil science, and industry. Phyllosilicates are used widely in many industries, ranging from cat litter, toxin-binding preparations for abdominal pain, and fillers and thickeners in paints, cosmetics, and pharmaceuticals.
The group's name comes from the Greek phýllon and means 'leaf'. Phyllosilicates are a class of minerals within the larger group of silicate minerals. Silicate minerals are characterized by their basic building block being the silica tetrahedron, which consists of a silicon atom surrounded by four oxygen atoms. In phyllosilicates, these tetrahedra are arranged in sheets. The basic structure of phyllosilicates involves forming sheets or layers, and these layers are typically composed of continuous networks of interconnected silica tetrahedra. The most common elements found in phyllosilicates include silicon, oxygen, hydrogen, and various metal cations. One of the defining characteristics of phyllosilicates is their sheet-like structure. This structure gives them properties such as a platy or flaky appearance, a characteristic cleavage along the sheets, and a tendency to absorb water and expand when hydrated. The sheets in phyllosilicates are held together by weak forces, making them prone to cleavage. In addition to clays, the group of phyllosilicates is also composed of micas, such as muscovite and biotite, which are minerals with excellent cleavage and are often used in various industrial applications and chlorite, a green mineral often found in metamorphic rocks. The unique properties of phyllosilicates make them important in various geological processes, including the weathering of rocks, soil formation, and the metamorphism of minerals.
Not all phyllosilicates have rheological properties, or not all have strong rheological properties. Some of the representatives of phyllosilicates, such as kaolinites-serpentines, in which kaolin clays can be found, are used as fillers. Although they have some influence on the rheology of the paint in which they are used, they are not rheological additives, just like talc, pyrophyllite or mica, which is also in the family of layered silicates. An overview classification of phyllosilicates is presented in Figure 1.
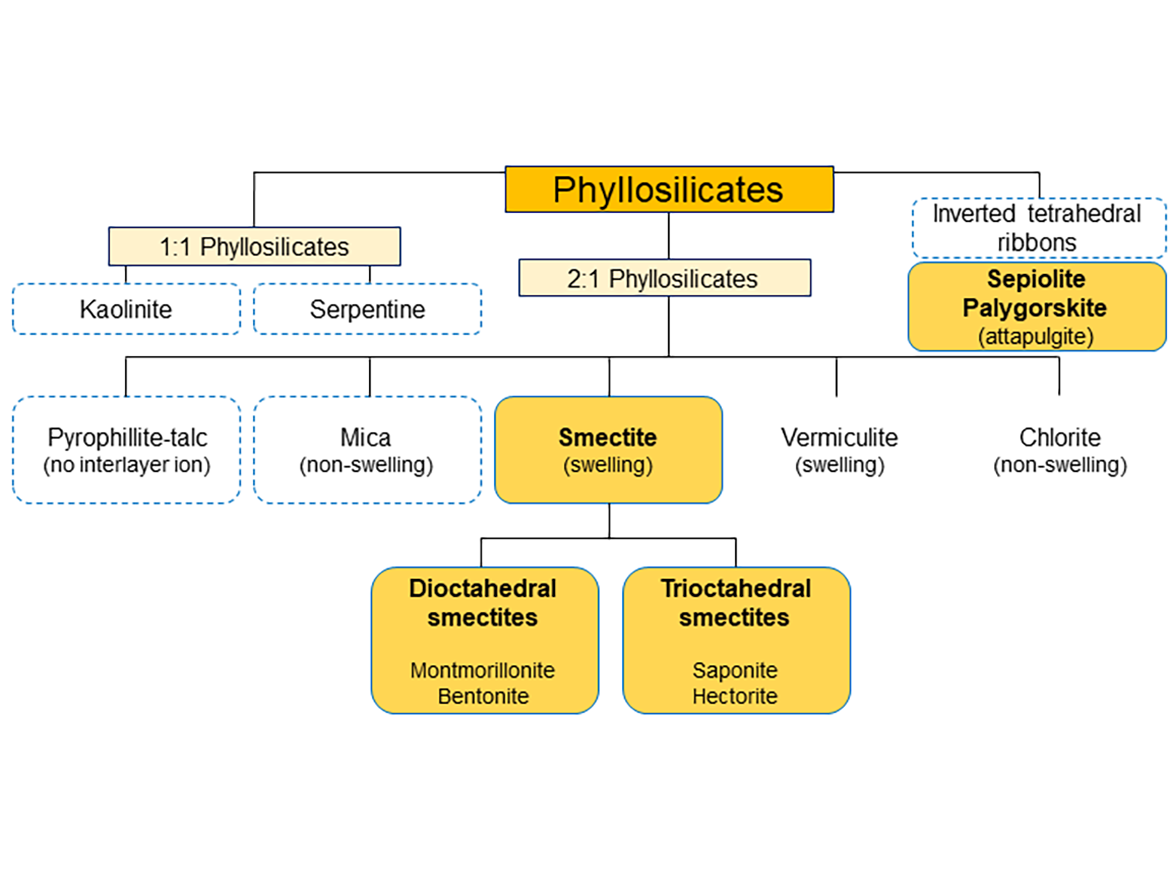
To build rheological properties, phyllosilicates with swelling properties are used, i.e. 2:1 phyllosilicates from the smectite group, as well as sepiolite and attapulgite. Notation 2:1 phyllosilicates meaning that it has two tetrahedral sheets of silica sandwiching a central octahedral sheet of alumina, and respectively one to one for 1:1 phyllosilicates.
Phyllosilicates are natural materials obtained from deposits. In the United States, bentonite, smectite, and attapulgite mines are widespread, but occur mainly in a few specific states. Here are some examples:
- Wyoming: This is one of the main bentonite mining sites in the United States. Regions such as the Big Horn Basin are particularly known for this resource.
- Montana: Bentonite is also mined in Montana, mainly around Fort Benton, which gives the mineral its name.
- Texas: Texas is another significant producer of bentonite. The Eagle Ford Shale area in Texas is one of the mining areas.
- Nevada: There are also bentonite mines in Nevada.
As for smectite and attapulgite, they can also be found in the same regions, as they are often found in geological formations where bentonite occurs. However, mining of these minerals may be less widespread and depends on specific locations and geological conditions.
Bentonite deposits are formed mainly by the action of water on volcanic rocks, such as volcanic tuffs or volcanic ash, which are rich in aluminosilicate minerals. The main mineral in bentonite is montmorillonite, which is a type of smectite. Weathering and erosion processes lead to the release of aluminosilicate minerals from the parent volcanic rocks, and then to their transport and deposition in places with appropriate hydrological conditions, where bentonite is formed.
Attapulgite deposits often occur in regions of strong chemical weathering where conditions favor the alteration of aluminosilicate minerals. They are formed by the reaction of water with silicate minerals in basalt rocks or other rocks containing magnesium or iron. This process transforms silicate minerals such as pyroxenes and amphibole into attapulgite.
Smectite deposits can form under a variety of conditions, but are often associated with weathering and degradation processes of rocks such as granites, limestone, or other rocks containing aluminosilicate minerals. Under the influence of water and chemical and biological factors, these rocks are degraded, which leads to the release and transformation of aluminosilicate minerals into smectite. Weathering conditions, humidity, pH, and the content of organic substances in the soil or rocks have a significant impact on the smectite formation process.
The group of phyllosilicates also includes sepiolite, which occurs in a fibrous or fibrous-scale form, called "earth powder" or "sea mineral" because it is often found in marine sediments.
Sepiolite often forms in environments where marine or freshwater sediments rich in minerals occur. One of the main processes of sepiolite formation is a chemical reaction occurring between igneous or metamorphic rocks containing silicates and water, which may contain magnesium and calcium compounds. Another process that can lead to the formation of sepiolite is the hydrothermal process, in which water rich in minerals penetrates rocks and chemical reactions occur, leading to the precipitation of sepiolite.
All these processes require time and appropriate geological and environmental conditions for bentonite, attapulgite, and smectite deposits to develop.
Phyllosilicates as Thickeners
Phyllosilicates are solid thickeners as off-white, cream, or gray pulverized powder or small flakes (Figure 2) with a moisture content of 6-13% and a density of approximately 12-24 lb/US gal.

Their properties of building a spatial network are the result of the swelling of layers (sheets), which, as a result of the action of shear forces and the time of their action, separate and swell. Phyllosilicates sheets form a "house of cards" that can be formed many times after the shear forces cease, and the time needed to return to the original viscosity structure causes the pseudoplastic nature of these thickeners to introduce more or less visible thixotropy (Figure 3).

Phyllosilicate thickeners in the hydrated gel form (Figure 4) can be added to the paint production process at the let-down stage, because in this form they are most effectively introduced into the suspension and separated into individual sheets. Adding phyllosilicate thickeners in powdered form during the grinding stage may be acceptable in certain circumstances, but is not recommended.

However, in order to properly prepare the gel and hydrate phyllosilicate clays, you must remember the four most important factors determining the effectiveness of hydration, which are:
- Hydration speed (shear forces);
- Hydration time at specific shear forces;
- Clay concentration in water;
- Type of water used for hydration.
The hydration process, which results in swelling of the space between the phyllosilicates sheets, occurs as a result of supplying energy to the suspension in the form of shear caused by the Cowles dissolver disc. For this process to take place effectively, an appropriate long exposure to energy (shear forces) is required, which causes the development of the viscosity of the phyllosilicate suspension to increase. This is illustrated in the example of an attapulgite suspension in Figure 5.
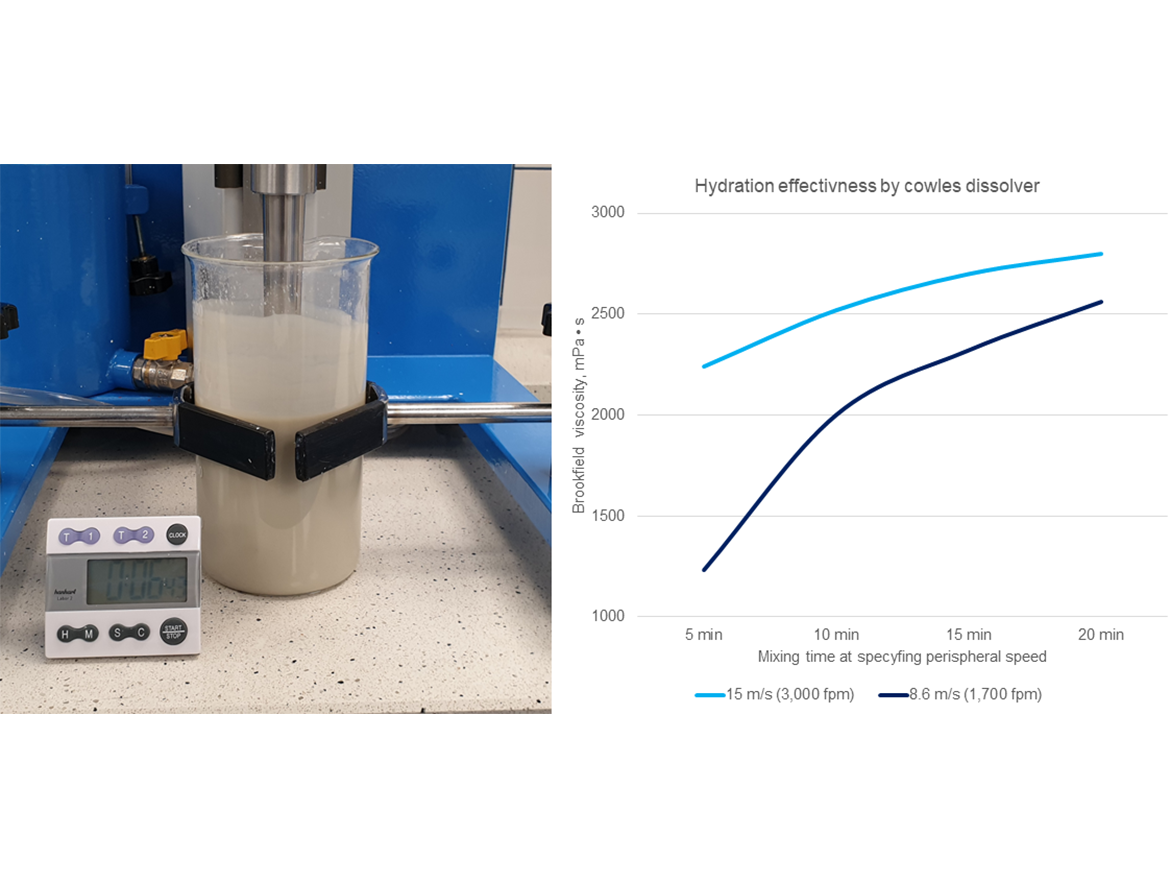
It is important to select the appropriate concentration of the thickener for gel hydration so that this process occurs effectively and the resulting gel is still pourable for further use in the production process of latex paints. However, there is no universal range of concentrations in which gels should be prepared for the hydration process to take place fully. Depending on the type of mineral, deposit, and characteristics of the sheets, different phyllosilicates can obtain different gel viscosities in different concentrations and thus different viscosity building efficiency.
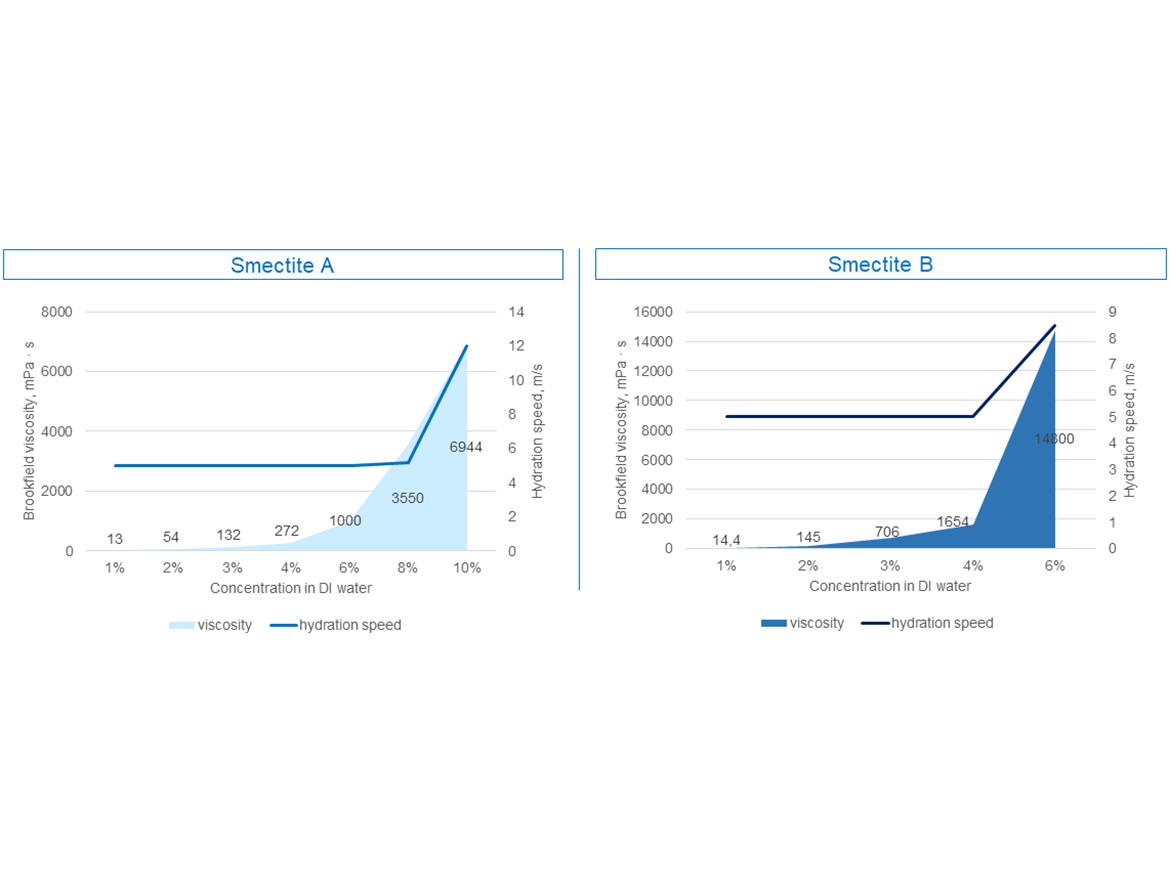
Figure 6 shows an example of two apparently similar smectites whose gels of different concentrations required the use of different energy in the form of shear forces to prepare gels of different viscosities. The flowability of these gels is shown in Video 1 and Video 2.
VIDEO 1 ǀ Pourability for smectite A with different gel concentrations.
VIDEO 2 ǀ Pourability for smectite B with different gel concentrations.
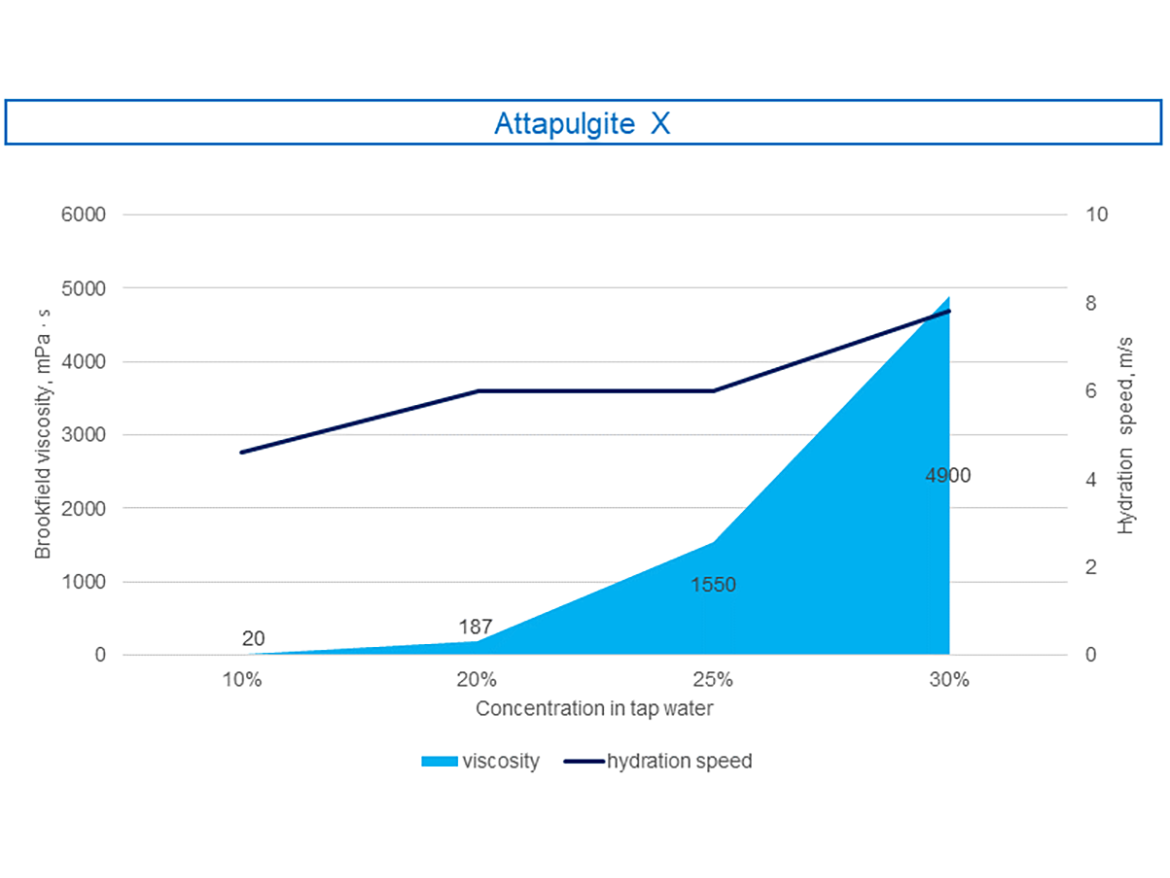
In turn, Figure 7 shows a graph showing the maximum loading and viscosity of the sample attapulgite. It can be seen that in the case of the presented attapulgite sample, the gel loading can reach up to 30%, however, this is associated with a significant increase in the viscosity and shear rate needed to prepare the hydrated gel. Video 3 shows the pourability of the prepared gels of the tested attapulgite.
The presented comparisons show how much different concentrations and viscosities were obtained to find the maximum loading at pourability and hydration at a specific peripheral speed of the dissolver disk. These differences result from the type of mineral, its susceptibility to hydration, the shape and size of the sheets, etc. These parameters must be determined each time for different phyllosilicate thickeners in order to prepare appropriate recommendations for the preparation of hydrated gels that can be used as thickeners in waterborne paints.
VIDEO 3 ǀ Pourability for sample attapulgite in various gel concentrations.
However, the exchange of ions that occurs during swelling between the sheets cannot be omitted during the hydration process. Water hardness also plays a role here and depends on the concentration of Ca2+ and Mg2+ cations. For some types of phyllosilicate thickeners, there is no significant difference between the degree of hydration and the development of their viscosity, as well as a further impact on the viscosity of paints regardless of the use of low-hardness (soft water, demineralized water) and hard water. In most cases, however, there is a significant impact of water hardness related to the ion exchange occurring during the hydration process, therefore the type of water also plays a role in the viscosity of phyllosilicate gels and paints in which they are used as thickeners, especially in terms of repeatability of paint production or transfer of production to another factory with access to water of a different hardness.
Figure 8 shows a comparison of the gel viscosity of an example attapulgite in demineralized water with a conductivity < 10 µS/cm and in hard tap water with a hardness of 30 °F (French scale)/300 ppm CaCO3 (3.0 mmol/L CaCO3) and a conductivity appoximately 1,100 µS/cm.
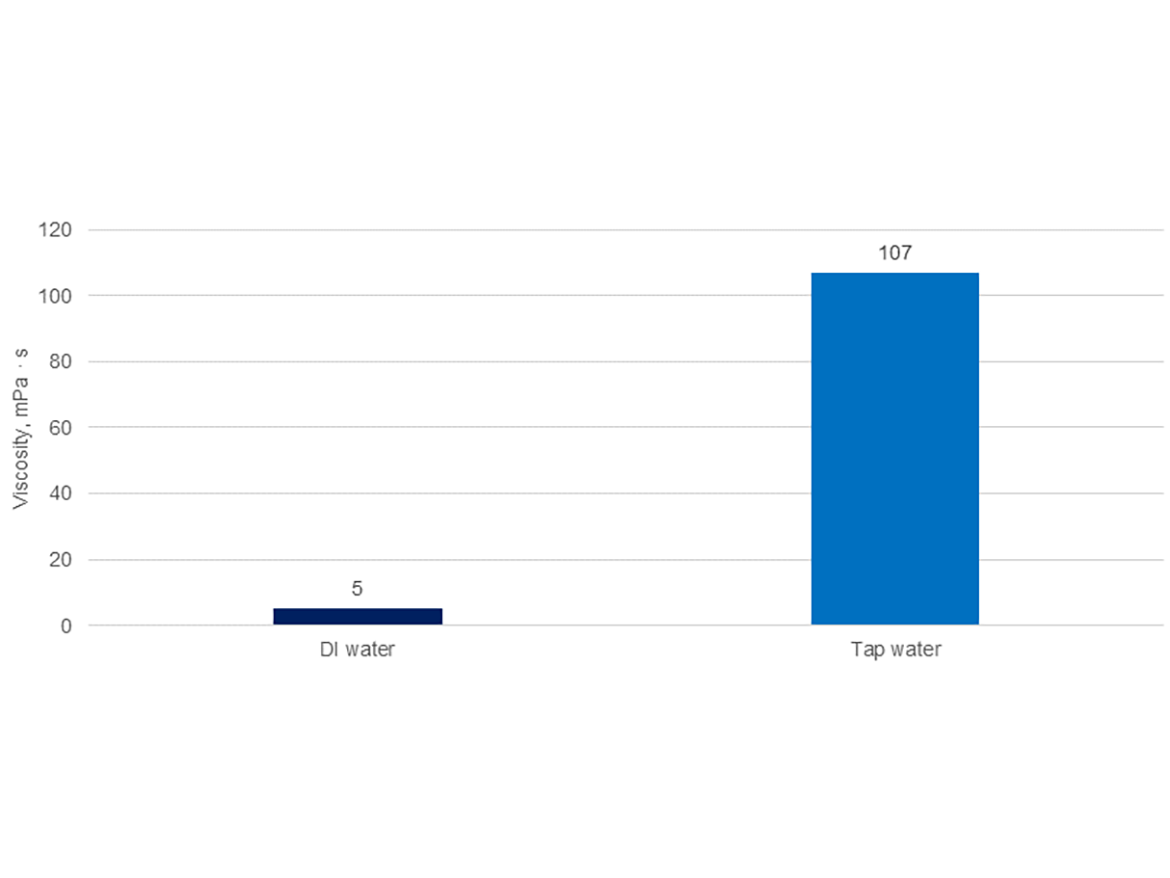
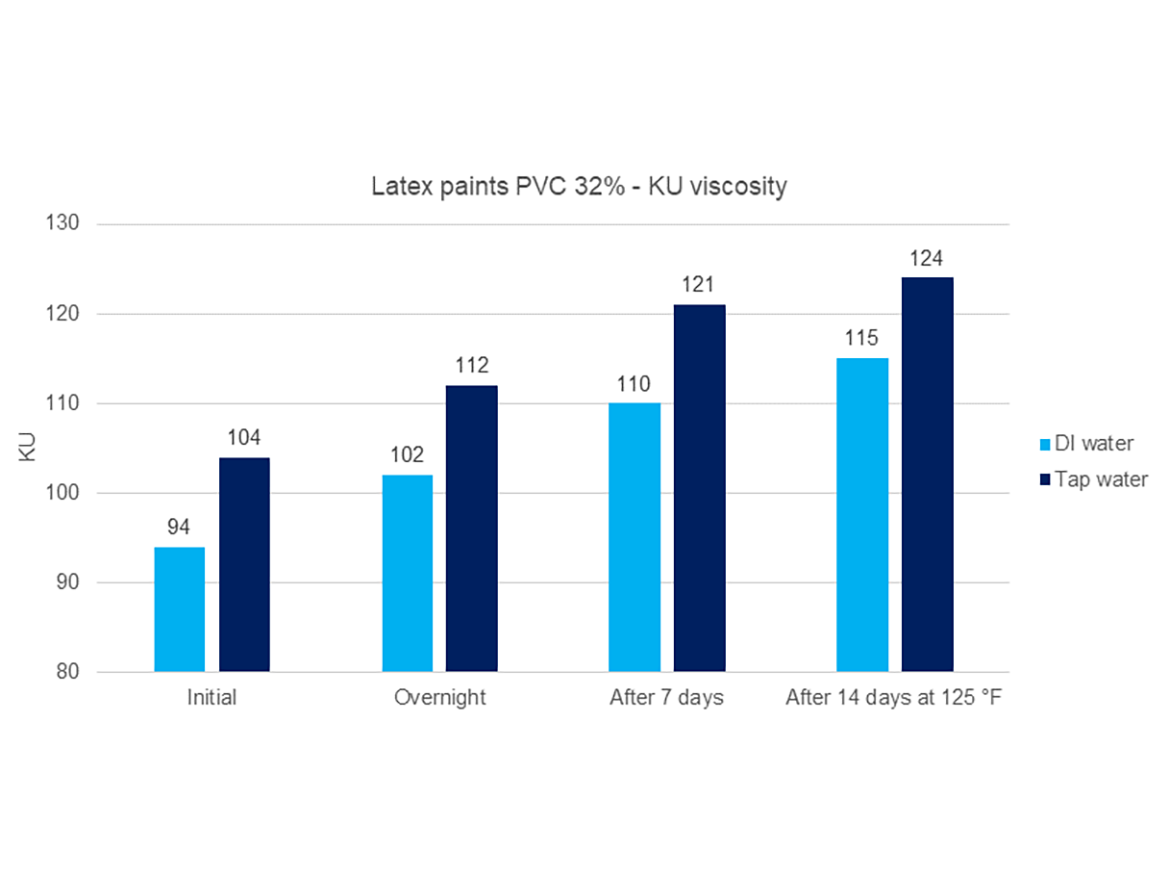
The gel sample in tap water (hard water) achieved a much higher level of hydration and viscosity, over 20 times higher than the sample in demineralized water. This proves the extremely significant impact of the presence of cations in water, which can be easily measured using a water hardness or conductivity test. This difference in the viscosity of the gel in water also affects the viscosity of the paints, as shown in Figure 9 by comparing the development of the viscosity of latex paint PVC 32% prepared on two gels of the same attapulgite, using demineralized water and hard tap water.
Experimental
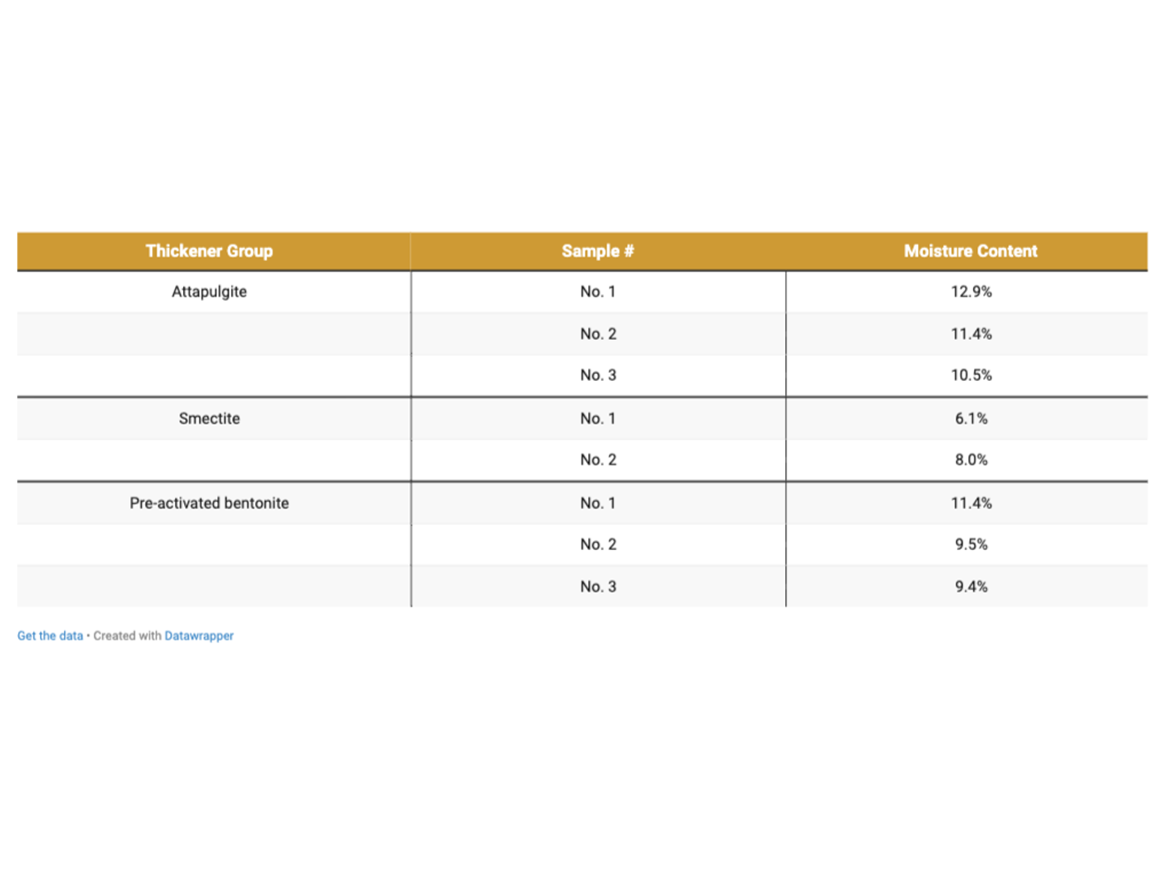
To better understand the influence of phyllosilicate thickeners on the rheological properties of latex paints, an experimental part was prepared to complement the research presented in the introductory part. The assumption of the experimental part was to perform application studies of various phyllosilicates thickeners (attapulgites, smectites, and bentonites) in the formulation of acrylic paints (PVC 34%) in order to determine their potential to build viscosity and secondary rheological properties. Phyllosilicates were used for the tests as in Table 1, and their moisture content was determined using a moisture analyzer in order to calculate the share of thickeners in the formulations in terms of active parts, excluding the moisture content.
The paint samples were prepared separately with each thickener, after preparing a gel of a specific concentration ensuring the maximum degree of hydration with good pourability. The paint samples were prepared with only the phyllosilicate thickeners used for the tests, which was intentional to show their influence on building rheological properties undisturbed by other rheological additives.
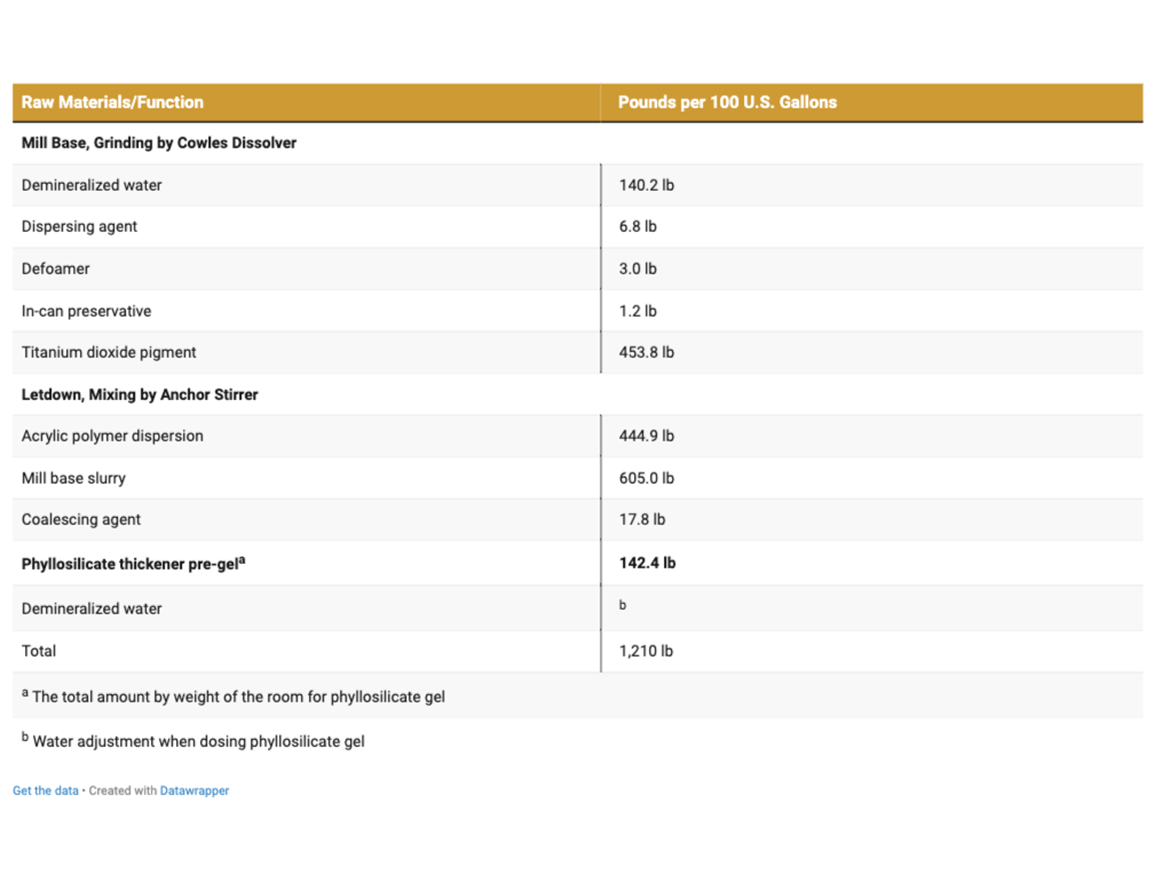
The dosage of individual phyllosilicate thickeners gels was set at a level ensuring viscosity in the range of 90-110 KU measured with a byko-visc DS Stormer viscometer after 7 days from the preparation of paints, which in practice meant dosing of gels in the range of 0.623 to 1.513 lb/U.S. gal of the finished paint.
The prepared paint samples were tested in accordance with the program indicated in Table 3. All tests were performed in accordance with ASTM procedures indicated in the table.
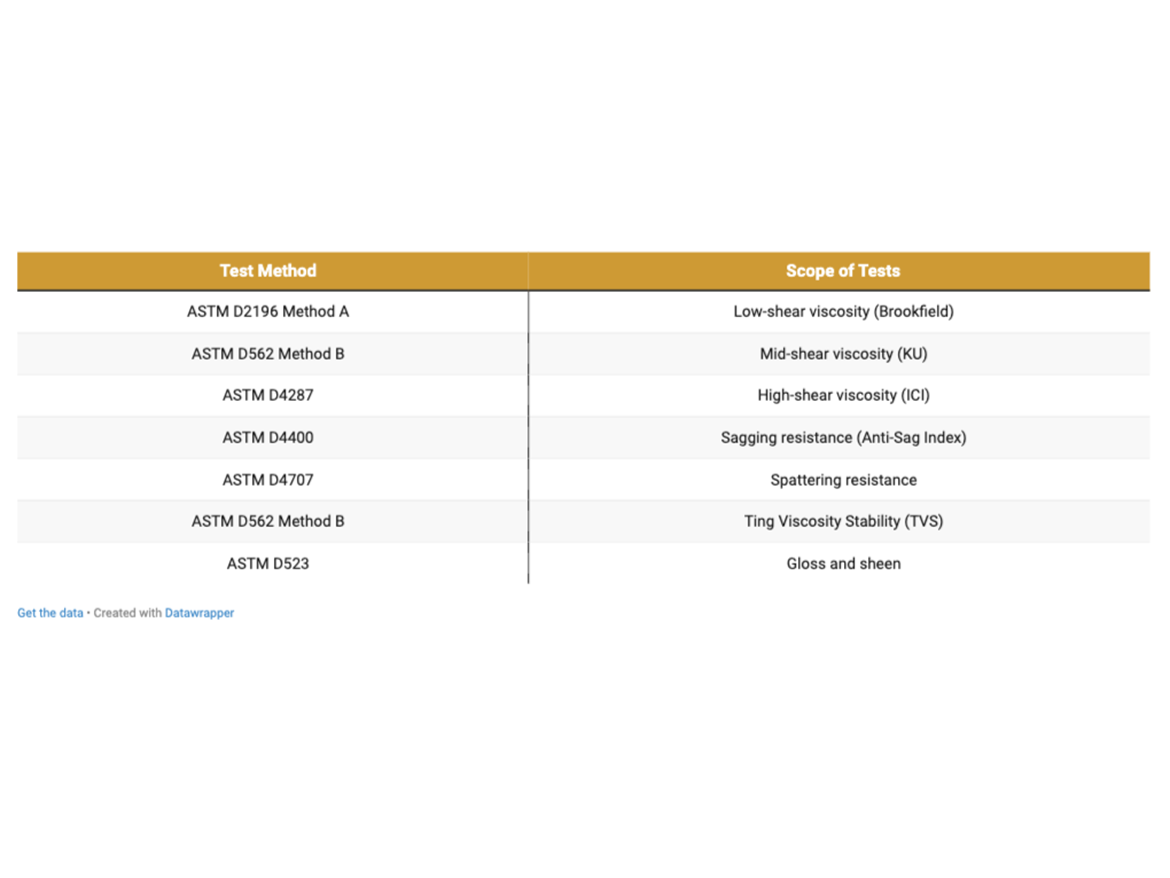
Viscosity
Figure 10 shows the results of viscosity measurements of low-shear (Brookfield viscometer), mid-shear (Stormer viscometer) to which the viscosity was adjusted, and high-shear (ICI CAP2000+L at 12,000 s-1). The obtained Brookfield viscosities depend on the viscosity level set to the KU viscosity and there are relationships between low-shear and mid-shear viscosity. Each of the thickeners used shows good effectiveness in modifying the viscosity in the los- and mid-shear area, depending on the initial viscosity setting in the mid-shear area. The high-shear viscosity measured using a cone-plate viscometer shows that these thickeners are not very effective in the ICI (high-shear) region, however, in the case of pre-activated bentonite No. 1, there is a clearly greater tendency to build viscosity, partly also in this area, as in the case of bentonite No. 3.
The results of viscosity measurements show that phyllosilicate thickeners are a good modifier of viscosity in the low- and mid-shear area, and the measurements in the high-shear area show that they can also be a good base for modifying and boosting the viscosity in the ICI area using other thickeners.
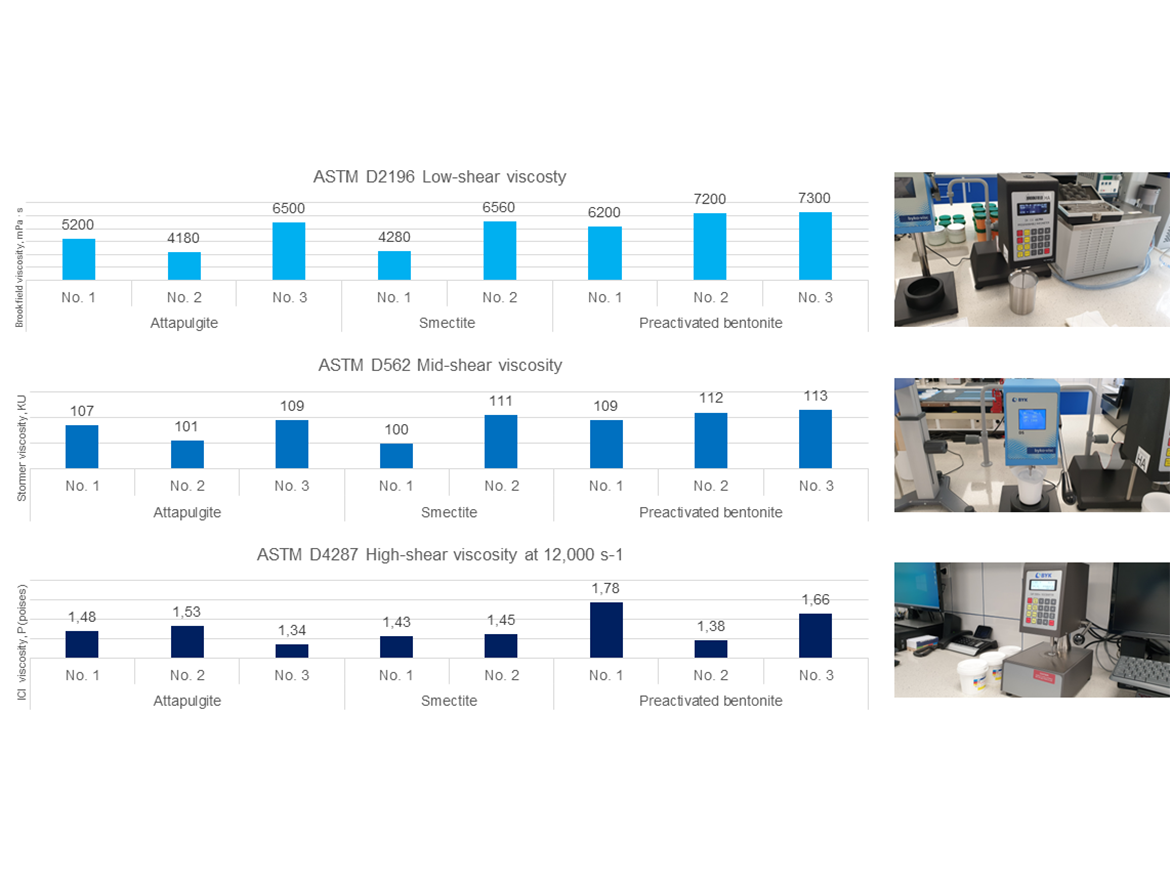
Resistance to Sagging
Resistance to sagging is a test from the secondary rheological parameters category that showed differences in the low-shear viscosity area that cannot be observed by viscosity/rheology measurements. As seen in Figure 11, thickeners introduce varying resistance to sagging as the Anti-Sag Index expressed in mils. The best resistance to sagging was demonstrated by attapulgite No. 3, and bentonites No. 2 and No. 3. The example of these results clearly shows the differences and the importance of performing additional tests to measure viscosity, which allow for the determination of rheological properties that are as important for practical application as sagging resistance, which allows for determining the influence of paint rheology on the thickness of the layer that can be applied to vertical surfaces without sagging.
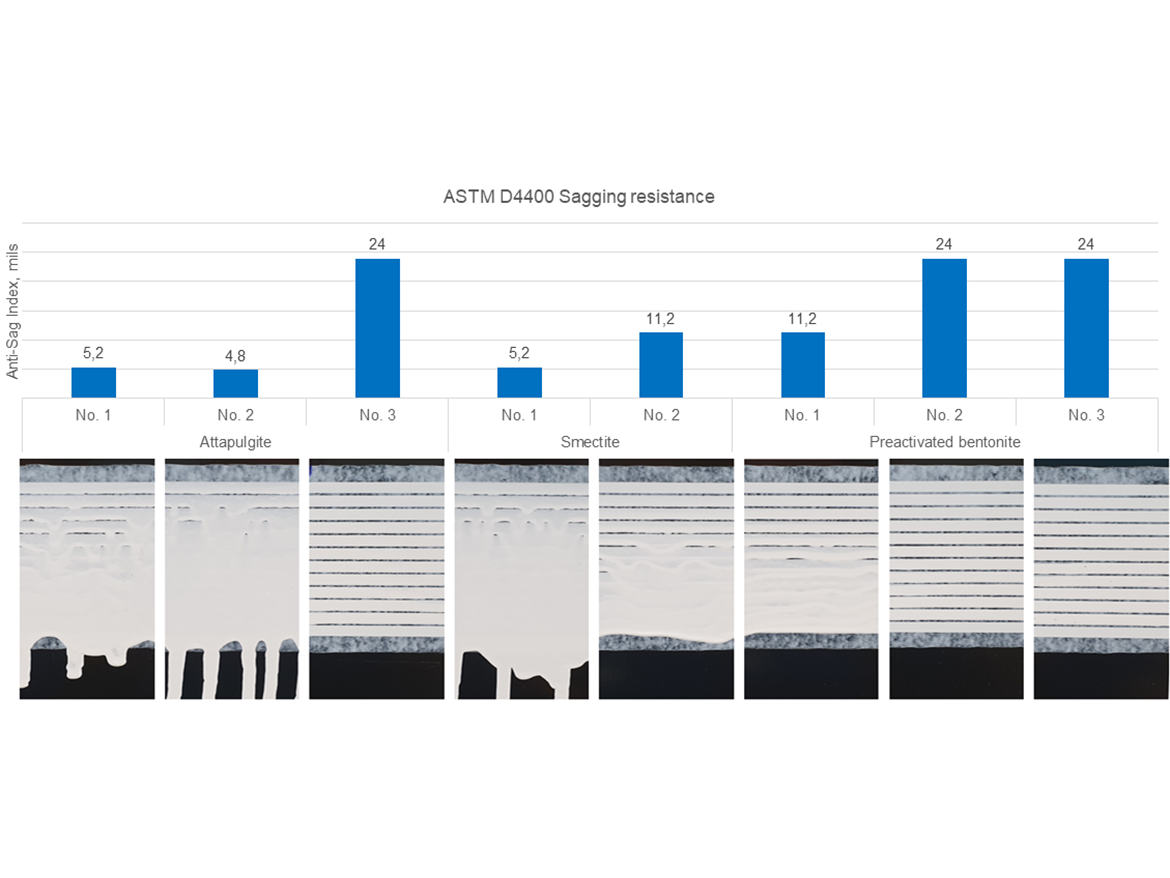
Spattering Resistance
The spattering test performed in accordance with ASTM D4707 is extremely valuable because it assesses the impact on the spattering of the paint itself and its rheological properties without taking into account the type of paint roller used for painting, as the test uses a special notched-spool roller strictly defined by the ASTM standard. Results are expressed on a scale of 0-10: 10=no spattering, and 0=the largest number of caught drops and paint spatters. Figure 12 shows the results along with photos of the substrates after the tests, showing the intensity of the spatter.
The best results were obtained for bentonite No. 3, and then for bentonite No. 1 and smectites No. 1 and No. 2. The remaining results also constitute a good starting point for modifying spattering resistance using other rheological additives. The worst result was achieved by bentonite No. 2, and in this example you can see that it is not possible to draw conclusions that if one bentonite showed a good result, each bentonite will behave similarly. Each phyllosilicate thickener is different and has a completely different tendency to influence rheological properties.
Tint Viscosity Stability
White paints, as we know, constitute an important segment of the market, but colored paints are the most important part of sales. Tintability is the ability of the base to accept pigment concentrates, which means adequate color repeatability, no rub-out caused by pigment flocculation, but also viscosity stability upon tinting. This parameter, called tint viscosity stability, is particularly important for point-of-sale bases, which are colored with pigments in the form of liquid concentrates in paint stores, and their dosage varies depending on the base and color. Figure 13 shows a graph of the changes after adding 7.6 fl oz/U.S. gal of PR168 colorant. Changes in viscosity upon tinting (KU viscosity drift) were adopted as a control parameter, where the acceptable viscosity range of 90-110 KU was marked, in which viscosity stability after tinting was expected. Unfortunately, this test showed the need to use additional thickeners to stabilize the viscosity upon tinting, because by adding a colorant, none of the tested thickeners was able to stabilize the viscosity in the desired range. Phyllosilicate thickeners are not good viscosity stabilizers after tinting and therefore it is necessary to use additional, often special and dedicated thickeners to stabilize tintability.

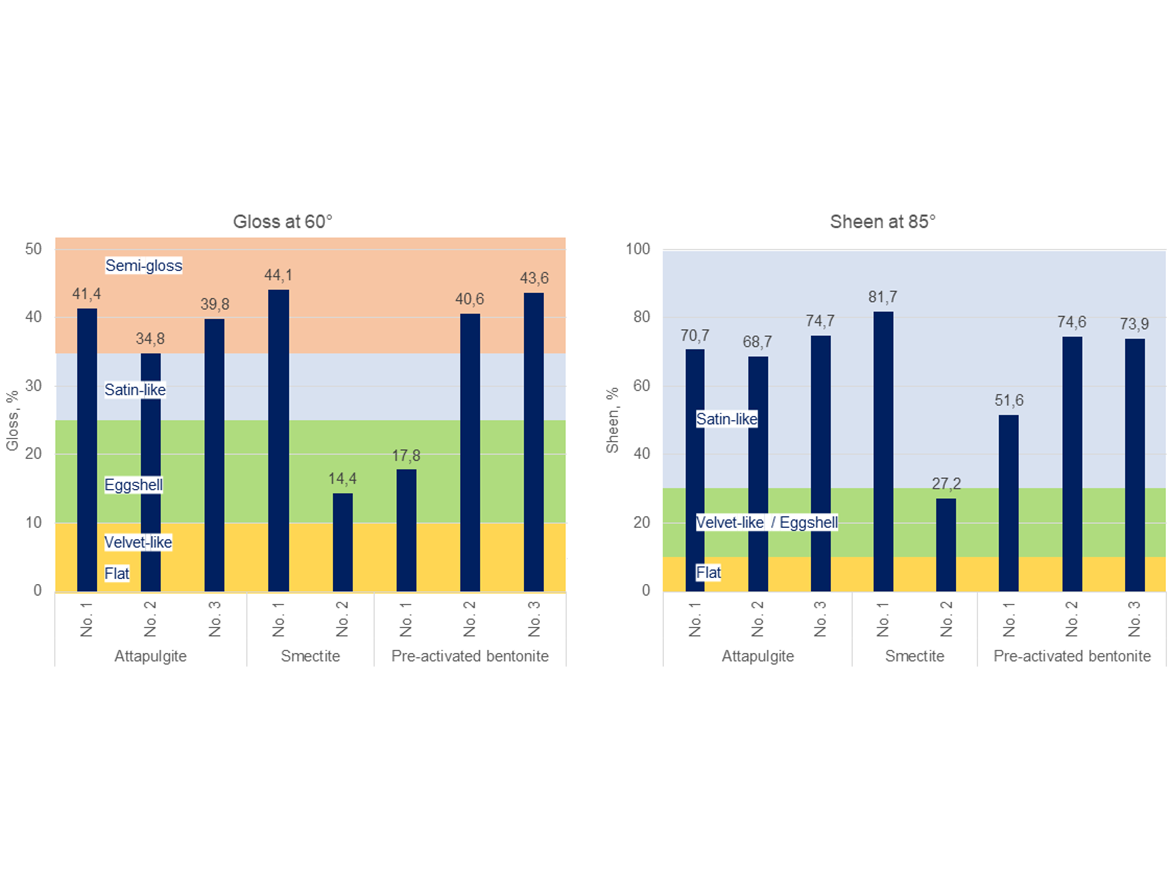
Gloss and Sheen
The last tested parameter, although not related to rheology, was gloss at 60° and sheen at 85° for coatings applied with tested paints containing phyllosilicates thickeners. However, it is important because these thickeners may reduce the gloss of the coatings. Figure 14 shows the results of gloss measurements and the classification of their degrees according to MPI's detailed performance standards. Due to the PVC and the composition of fillers and pigments, the initial gloss at 60° is semi-gloss, which was achieved for paint samples with attapulgite thickeners No. 1 and No. 3, smectite No. 1, and bentonites No. 2 and No. 3. Paint coating containing attapulgite No. 2 achieved a satin-like gloss level, and paints containing smectite No. 2 and bentonite No. 1 create coatings with an eggshell finish. These results show how drastic an impact phyllosilicate thickeners can have on the gloss, which can be consciously used to build a specific level of matting, or use only those thickeners that do not affect the matting, in the case of the intention to create a coating with an increased level of gloss.
The situation is similar with sheen at 85°, although here all samples except smectite No. 2 showed a degree of sheen satin-like, and the mentioned smectite No. 2 is in the upper limit of the velvet-like/eggshell classification in terms of gloss. This is extremely important information for both the formulator and suppliers of such thickeners as an advantage for use in the formulation of paints with varying gloss levels, as well as an extremely valuable tip when formulating higher gloss/sheen paints to provide formulators with data on which thickeners do not reduce the gloss.
Summary
As you can see, phyllosilicate thickeners are not easy additives to use in formulations and require knowledge, experience, and guidance to obtain the best effectiveness from them. Their ability to build rheological properties, adjust gloss, as well as boost rheological properties together with other thickeners make them extremely interesting rheological additives, especially in architectural latex paints. Providing phyllosilicate thickeners for specific types requires meticulous research and analysis of the impact on rheological properties. I presented these details in the test results, as well as the next step with the analysis of the impact on rheological properties in real-life formulations with HEUR/HEC or HASE thickeners, which are used together with bentonites, smectites, or attapulgites to create rheologically perfect latex paints. Providing such data not only facilitates the introduction of phyllosilicate thickeners to the market, but also makes the formulation of paints using them easier.
References
1 Palasz, A. Phyllosilicate Thickeners as One Family of Clays in Each of Them is Different, Waterborne Symposium 2024, New Orleans, LA.
This article was prepared on the basis of the paper "Phyllosilicate Thickeners as One Family of Clays in Each of Them is Different"1 author Artur Palasz, presented during the Waterborne Symposium 2024, New Orleans, LA, US and awarded the PCI Technical Excellence Award.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









