Materials and Methods Enabling Robust Matte Appearance

Image courtesy of PPG.
Gloss of an applied coating is a key appearance and styling feature used in decorative and functional surfaces. In military and related applications, low-gloss serves a tactical purpose for reduced detectability. In many other applications, including automotive and related coatings, low gloss is a small but growing portion of the market as a styling feature. Low-gloss appearance is often utilized as a premium feature.
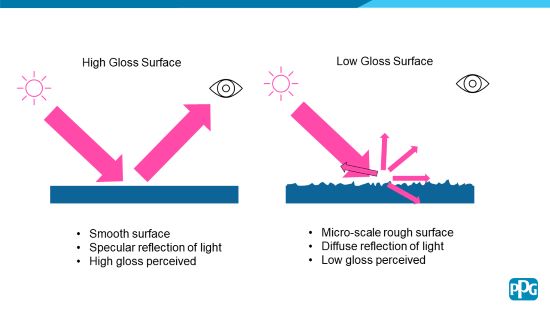
It is helpful to understand the mechanism of the appearance of low-gloss coatings. To achieve a low-gloss appearance, it is required that a micro-scale roughness is achieved at the coating surface that will scatter light in a diffuse manner, thus giving the desired low-gloss appearance. In contrast, a high-gloss appearance is achieved with a smooth surface that results in a largely specular reflection of light, giving a high gloss appearance (Figure 1).
FIGURE 1 | Mechanism of matte appearance.
The micro-scale roughness that is required for low gloss appearance is often attained through the use of matting agents, although other approaches also exist. The matting agents are typically particles that can be inorganic or organic, with the key being that they are insoluble in the binder such that their shape is maintained to create surface roughness (Figure 2). Silica is a common inorganic matting agent used in coatings. The exact size and concentration of the matting agent are key formulation factors that contribute to the observed gloss level of the applied coating.
FIGURE 2 | Matting agents cause micro-scale surface roughness.

One common issue with low gloss coatings can be the tendency for the gloss to increase with use and exposure over time. This increase in gloss of a low gloss coating is called burnishing and leads to non-uniform appearance due to variation in gloss across the coating surface. Burnishing can occur when wear of the surface causes loss of the matting agent at the surface and effectively creates a localized binder enrichment. This localized binder rich area can effectively be polished to some extent, increasing the gloss of that area, often in a non-uniform and undesirable manner. Coatings that can resist burnishing are desired to enable the longevity of the desired appearance. Two different approaches to achieving burnish resistance matte appearance are presented herein.
Experimental
Amine Functional Silica Matting Agent
Amine functional silica1 was prepared using Hi-Sil® 2000P, a precipitated silica product of PPG Industries, that was milled and classified to a volume median particle size (measured by Beckman Coulter LS 230 instrument) of 10 micron and a maximum particle size of 22.5 micron. This silica was treated with 16 wt% of 3-triethoxysilylpropylamine by blending it in a Waring blender continuously with silane pumped at uniform rate for five minutes with a Masterflex pump fitted with Viton tubing. The uniform blend was subjected to 120 °C for two hours in a convection oven.
Formulas 1 and 2 demonstrate the preparation of burnish resistant matte clear coats using amine functional silica. The coatings were prepared by first mixing the separate packs of ingredients as listed in Table 1, and then combining the packs immediately prior to application to the substrates.
TABLE 1 | Formula 1 and 2.
Polysiloxane/Silica Dispersion Matting Agent
First, a polysiloxane resin2 was prepared via the hydrosilylation of pentasiloxane with an approximate degree of polymerization of 3 to 4, i.e., (Si-O)3 to (Si-O)4. The polysiloxane polyol was prepared from the following mixture of ingredients in Table 2.
TABLE 2 | Polysiloxane resin preparation.
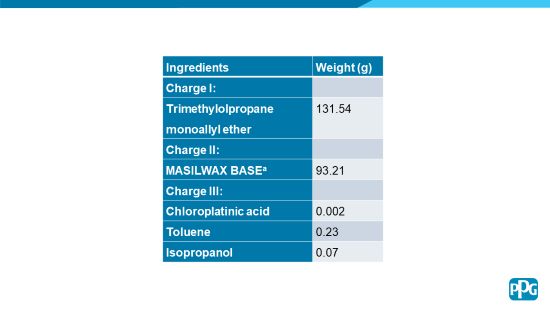
To a suitable reaction vessel equipped with a means for maintaining a nitrogen blanket, Charge I from Table 2 and an amount of sodium bicarbonate equivalent to 20 to 25 ppm of total monomer solids was added at ambient conditions and the temperature was gradually increased to 75 °C under a nitrogen blanket. At that temperature, 5.0% of Charge II from Table 2 was added under agitation, followed by the addition of Charge III from Table 2, equivalent to 10 ppm of active platinum based on total monomer solids. The reaction was then allowed to exotherm to 95°C at which time the remainder of Charge II was added at a rate such that the temperature did not exceed 95 °C. After completion of this addition, the reaction temperature was maintained at 95°C and monitored by infrared spectroscopy for disappearance of the silicon hydride absorption band (Si-H, 2150 cm-1).
To prepare a polysiloxane/silica dispersion2, 25 g of ACEMATT® TS100 (available from Evonik Industries AG) was mixed with 250 g of methanol in a four-neck round bottom flask equipped with a mechanical stirrer. While stirring, HCl acid was added until the viscosity of the mixture was markedly reduced and the pH was less than 1. After stirring for one hour the mixture was filtered using a Buchner funnel, rinsed with methanol and then allowed to dry at 120 °F (49 °C) overnight. The protonated silica was then mixed with 256 g methanol and stirred for 30 min in a four-neck round bottom flask equipped with a mechanical stirrer, temperature probe, reflux condenser, addition funnel, vacuum outlet and a nitrogen inlet. Then the 144 g of the polysiloxane polyol, as described above, was added to the methanol/silica mixture. Upon completion of the polysiloxane addition 163.38 g of methyl isobutyl ketone was added and the entire mixture was stirred for 45 min. The mixture was then heated to 30°C and vacuum applied to remove methanol. After the desired amount of methanol was removed the mixture was heated to 80 °C for one hour.
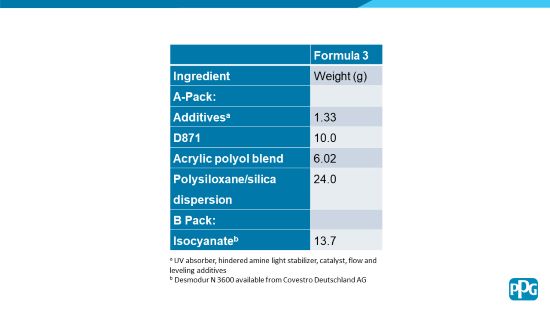
The dispersion was then incorporated into a clear coat formulation, prepared by first mixing the separate packs of ingredients as listed in Table 3, and then combining the packs immediately prior to application to the substrates.
TABLE 3 | Formula 3.
Coating Application
The matte clear coats of Formulas 1 through 3 were spray applied to a pigmented basecoat to form color-plus-clear composite coatings over primed electrocoated steel panels. The panels used were ACT cold roll steel panels 4 in x 12 in (10.16 cm by 30.48 cm) with ED6060 electrocoat available from ACT Laboratories, Inc. Separate panels were coated with an ENVIROBASE® High Performance (EHP) pigmented waterborne basecoat, available from PPG Industries, Inc. Black EHP T407 was hand sprayed using a SATAjet 3000 with WSB fluid nozzle at ambient temperature (about 70 °F (21 °C)). A dry film thickness of about 0.3 to 0.8 mils (about 7 to 20 micrometers) was targeted for the basecoat. The basecoat panels were allowed to flash at ambient temperature (about 70 °F (21 °C)) for at least 15 minutes prior to clearcoat application.
The clear coat formulas were each hand sprayed using a Devilbiss GTi HVLP spray gun to a base-coated panel at ambient temperature in two coats with an ambient flash between applications. Clearcoats were targeted for a 1.5 to 2.5 mils (about 38 to 64 micrometers) dry film thickness. All coatings were allowed to cure at ambient temperature or air flash for about 20 minutes before being baked. The bake was for thirty minutes at 140 °F (60 °C). Seven days after clearcoat application, the coated panels were subjected to Dry Abrasion Test Method One and Two and Wet Abrasion Test Methods One and Two to determine burnish resistance.
Testing Methods
Each of the Dry Abrasion Test Methods One and Two are carried out such that the coating is linearly scratched with a weighted abrasive paper for ten double rubs using an Atlas AATCC Crockmeter, Model CM-5, available from Atlas Electric Devices Company of Chicago, Ill. The abrasive paper used is 3M 281Q WETORDRY™ PRODUCTION™ two and nine micron polishing paper sheets for Dry Abrasion Test Methods One and Two, respectively, which are commercially available from 3M Company of St. Paul, Minn. In the Dry Abrasion Tests One and Two, a cured coating on a substrate is subjected to testing by first measuring the 85° gloss of the coating (“original gloss”). The coating is then subjected to DRY ABRASION TESTS ONE and TWO, and afterward, the 85° gloss is again measured.
Each of the Wet Abrasion Test Methods One and Two corresponds, respectively, to the Amtec-Kistler Car Wash Test DIN 55668, run at 10 or 40 cycles, respectively. In the Wet Abrasion Tests One and Two, a cured coating on a substrate is subjected to testing by first measuring the 85° gloss of the coating (“original gloss”). The coating is then subjected to the Amtec-Kistler Car Wash Test DIN 55668, run at 10 or 40 cycles, and afterward, the 85° gloss is again measured.
Results and Discussion
Figure 3 below illustrates the Wet Abrasion Test Method results and Figure 4 illustrates the Dry Abrasion Test Method results for the Formulas 1 through 3. A commercially available control matte clear coating is included for reference and comparison.
FIGURE 3 | Wet abrasion testing results of Formulas 1 through 3.
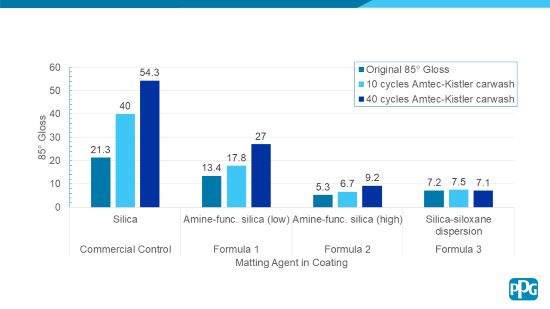
FIGURE 4 | Dry abrasion testing results of Formulas 1 through 3.
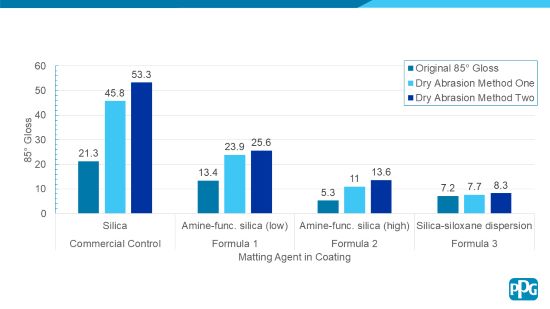
As can be seen in Figures 3 and 4, the Formulas 1 through 3 demonstrate excellent burnish resistance via two different matting agent approaches. The coatings show a gloss increase of less than 5 gloss units for Wet Abrasion Test Method One. Also, the coatings show a gloss increase of 12.2 gloss units or less for Dry Abrasion Test Method Two.
Burnishing of matte coatings occurs when the coating is effectively polished (increased gloss) in the course of its use, leading to undesirable variations in gloss across the coating surface. One mode that this occurs includes the loss of the matting agent at the surface, leaving a local region of binder rich coating that is more susceptible to polishing.
The use of an amine functional group on the silica particles provides a means for covalent bonding of the matting agent and the coating binder. It is hypothesized that covalent bonding of the matting agent provides a greater barrier to remove the matting agent from the coating. If the matting agent persists in the coating surface there is less formation of localized binder rich region that would be susceptible to polishing, thus creating a burnish resistant surface. In the polysiloxane/silica dispersion another mode of burnish resistance is employed. The polysiloxane resin has been used in high gloss clearcoats as a means of scratch resistance. It is hypothesized that the while the micron sized silica provides a textured surface for light scattering for a matte appearance, the polysiloxane resin is providing scratch or polishing resistance, thus enabling minimal changes in gloss during testing or use. This method showed exceptional burnish resistance with almost no change in gloss during testing.
Conclusions
Two separate methods for achieving burnish resistance coatings have been demonstrated herein. Amine functional silica is an approach that fits easily into existing manufacturing processes and provides desirable burnish resistance. The polysiloxane/silica dispersion delivers exceptional burnish resistance but requires a more complicated process to manufacture.
Acknowledgements
Thank you to all of those that helped with this work in some way. A special thanks to Rich Winters who contributed to the polysiloxane/silica dispersion approach. Additional contributors to this article include Lawrence Anderson and Stephen Thomas, PPG Coatings Innovation Center, Allison Park, Pennsylvania; and Shantilal Mohnot, PPG Monroeville Business and Technology Center, Monroeville, Pennsylvania.
References
1 Moravek, S. J.; Mohnot, S.; Thomas, S. J. Methods of Improving Burnish Resistance Using Curable Film-Forming Compositions Demonstrating Burnish Resistance and Low Gloss. U.S. Patent 9,969,900. May 15, 2018.
2 Moravek, S. J.; Anderson, L. G. Curable Film-Forming Compositions Demonstrating Burnish Resistance and Low Gloss, and Methods of Improving Burnish Resistance of a Substrate. U.S. Patent 10,093,829. Oct. 9, 2018.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







