New Additive Provides Improved Graffiti Resistance and an Easy-To-Clean Surface

Highly functionalized surface additives can provide industrial coatings and printing inks with a durable, lubricant surface that is easy to clean. Siloxane-based additives play a major role in the performance of many of these high-performance formulations. The use of organo-modified polysiloxanes in surface additives is growing beyond the typical applications in wetting agents and defoamers. Due to their unique interfacial activity, modified siloxanes are invaluable ingredients that promote smooth film surfaces by improving leveling and minimizing craters. This also results in functional surface properties such as slip or easy-to-clean effects.1 Details of the chemistry and resulting performance attributes of a new, highly compatible, organo-modified, highly functional siloxane-based ETC additive (OHSA) are presented here.
Design Elements of a Surface-Active Additive
The versatility of modifications to the polysiloxane base unit enables fine-tuning of properties that meet the challenges of multiple applications and coating systems. The degree of propoxylation (PO), ethoxylation (EO), and polysiloxane content can be varied in almost any manner by targeted synthesis (Figure 1).
FIGURE 1 ǀ Structural combination possibilities for creating polysiloxane-containing additives with varying modes of action in the system.

When increasing the hydrophobicity of the additive by increasing the number of PO units, the incompatibility in the aqueous system increases, which results in a defoaming effect. Conversely, increased amounts of EO improve compatibility by making the additive more hydrophilic. Accordingly, the ratio of PO and EO can be used to precisely adjust compatibility in the system.
The polysiloxane component of the additive influences the interfacial effect. As the polysiloxane molecular weight and its proportion to total additive molecular weight increase, the additive molecules will orient more strongly in a coating at the coating/air interface. This enables the additive to align at the surface and influence numerous surface properties. These variation possibilities allow a perfectly balanced adjustment of compatibility, insolubility, and interfacial activity for each system and application.2
Once the desired performance profile has been set, the additive can be additionally functionalized with anchor groups. Choosing anchor groups that interact supramolecularly with the coating binder and that bind intermolecularly within the system can minimize migration of the additive over time, with the surface properties and ETC performance remaining persistent even after repeated cleaning cycles.
Anchor Group Chemistry of OHSA
The sustained long-term performance of the OHSA surface-active additive is achieved through a hydrophilic anchor function that binds to the system via intermolecular interaction. A distinction is made here between non-directed interactions such as van der Waals forces and directed interactions such as hydrogen bonds. While the former is very weakly pronounced, the latter show comparatively large bond strengths and can be in the range of a covalent bond.3 The hydrogen bonds are usually formed by interactions of a covalently bonded, positively polarized hydrogen atom and a free electron pair of an electronegative atom such as oxygen. In the case of OHSA, the anchor group is hydroxy-functionalized and able to form hydrogen bonds with the hydrophilic components of the binder and, as such, can anchor permanently in the system (Figure 2, left). The hydroxyl anchors also increase the compatibility of OHSA in aqueous systems.
FIGURE 2 ǀ Two types of bonding exhibited by the OHSA surface-active additive. Left: Hydrogen bonding between the hydrophilic anchor group (dark green) of the additive (yellow-green) and the binder (blue). Right: Covalent bonding of the additive hydroxyl groups to an isocyanate group of the hardener in a two-part polyurethane (2K PU) system to form a stable urethane group.

OHSA Testing in an Aqueous Overprint Varnish and a Two-Part Industrial Coating
OHSA was tested in two types of systems. The first is an aqueous overprint varnish (OPV) formula based on a flexible polyurethane dispersion (PUD), similar to coatings for graphic arts applications. These applications tend to be too low in coat weight to provide significant ETC or anti-graffiti effects, so the resulting test proofs were assessed only for leveling and surface defects, and for effect of the additive on COF. The second test system is a two-part polyurethane (2K PU) system, which was applied at typical industrial coat weights and tested for multiple attributes related to ETC performance.
Samples of the PUD OPV were made with 3% OHSA and 3% of a standard surface additive, which were compared to a blank sample with no additive. A small amount of blue colorant was added to each sample to facilitate visual assessment of the OPV surface after application. After application to Leneta test charts at a dry film thickness of 6 μm, several test OPV formulas were visually assessed for surface quality and uniformity. COF of the proofs was measured with a ZwickRoell zwickiLine Z2.5 materials testing machine with a 1-kg weight.
Variations of the 2K PU industrial coating were made with 3% OHSA, 3% of the standard surface additive, and no additive (blank). The test formulas were applied to Q-Panels with a high-volume low-pressure (HVLP) spray gun at a wet film thickness of 125 μm for the signature removability and cyclic cleaning tests. For the spray paint tape release test, the formulas were applied to Q-Panels with a wire-wound rod at a wet film thickness of 100 μm. The dried panels were visually assessed and evaluated for various release qualities.
The signature removability test is conducted by writing on the dried coating with permanent marker or paint pen, then wiping with cleaning fluid within minutes of marker or paint application, allowing the application to be dried. The cleaned coating surface is examined for signs of marker staining or shadowing and rated on a scale from 1 to 10, with 10 indicating complete marker removal and no staining.
The cyclic cleaning test consists of writing on the cured coating surface with a permanent marker pen over multiple lines, in this case over four lines. After the marker has dried, for the first cleaning cycle, all four written lines are removed as completely as possible with isopropanol on a white cellulose pad. The cleaned coating surface is visually assessed for marker residue or shadowing. The second through fourth lines are then overwritten with the permanent marker, dried, then cleaned again. The third and fourth lines are then overwritten, dried, and cleaned, and finally only the fourth line is overwritten, dried, and cleaned again. In this way, the first line shows any residual writing left after one cleaning cycle, the second line shows the results after two cleaning cycles, and so on. Visual assessments are rated from 1 to 10, with 10 indicating no staining of the coating surface after marker removal.
For the tape release test, solvent spray paint is applied to dried coated panels. After the paints are allowed to dry, strips of clear PVC packaging tape are pressed firmly onto the painted surface then quickly removed. The panels are assessed for completeness of paint removal from the coated surface and onto the tape.
Results and Discussion
Results in the Aqueous PUD OPV
In the OPV tests, the standard surface additive could not be finely dispersed in the aqueous PUD, resulting in visible coarse emulsion droplets after the OPV sample was applied to the test chart. These concentrated areas of extremely low surface energy create a non-uniform coated surface that is especially evident in thin film applications such as this (Figure 3). The OHSA, on the other hand, can distribute itself in the OPV without interference due to its structural ability to form pronounced intermolecular interactions, enabling the formation of a defect-free surface. The polysiloxane content of both the standard surface additive and OHSA orient them to the OPV surface, imparting a strong slip-release effect and improvement in scratch and abrasion resistance. This is also shown in Figure 3, where the blank sample is visibly abraded by the three contact points of the 1-kg COF testing sled while no mechanical injury is evident in the 3% standard surface additive or 3% OHSA samples.
FIGURE 3 ǀ Application of water-based OPV samples made with 3% standard surface additive, 3% OHSA, and a blank with no additive on black/white test chart, 6 µm dry film thickness. The poor dispersibility of the standard surface additive results in a non-uniform surface, but the polysiloxane content still imparts scratch and abrasion resistance for this and the OHSA-containing OPV samples relative to the blank.
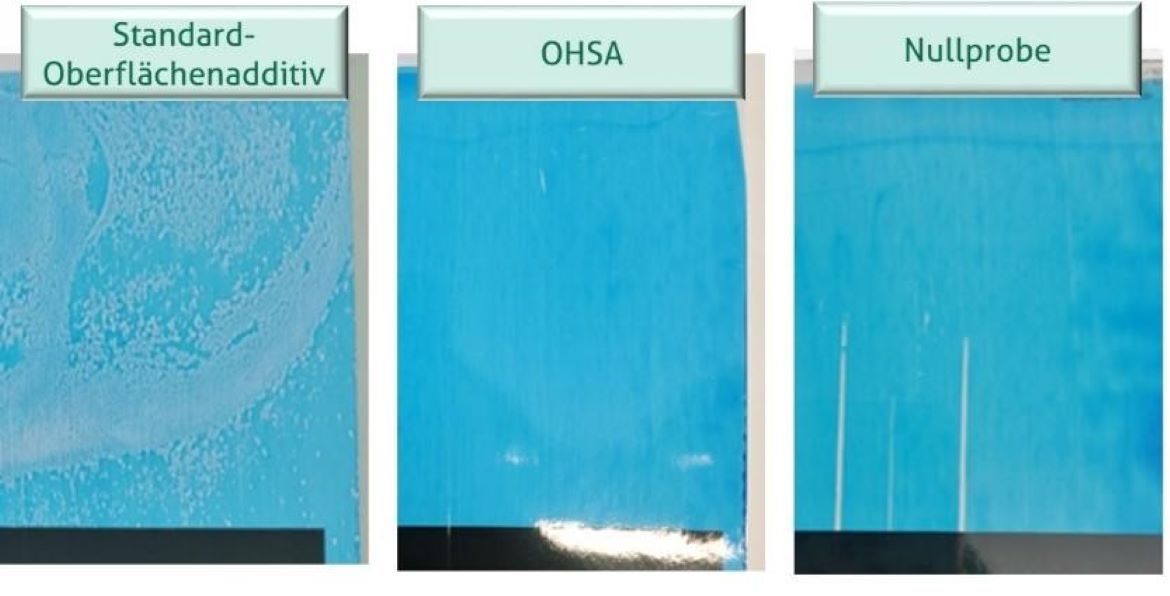
Table 1 and Figure 4 show that the proof of the blank OPV has a dynamic frictional force (FD) of 5.46 N. The 1.5% OHSA sample has considerably more surface slip and is approximately 2 N lower (FD = 3.46 N). This value is further lowered by only about 0.5 N when the OHSA is increased to 3% (FD = 2.94 N), indicating that 1.5% OHSA is sufficient for surface effects in the OPV.
TABLE 1 ǀ COF data for OPV samples containing no additive (blank), and 1% and 3% OHSA.

FIGURE 4 ǀ COF graph for OPV samples containing no additive (blank), and 1.5% and 3% OHSA.

The PUD binder in the test OPV formula has already undergone the reaction step of diol-isocyanate addition to form a covalent polyurethane bond. Even so, strongly polar or even ionic groups such as sulfonate groups are present on the binder polymer backbone. These are used for permanent hydrophilic modification and easy dispersion of the binder in water to achieve the final formulation form and represent ideal interaction partners.
Results in the Two-Part Industrial Coating
Table 2 shows results of comparative tests conducted on 2K PU coating samples containing no additive (blank), 3% of the same standard surface additive previously tested in the aqueous OPV, and 3% OHSA. As with the OPV tests, the standard surface additive made a 2K PU coating with poor surface quality, in this case exhibiting turbidity and orange-peel defects. It did, however, show considerably improved performance against the blank sample in the signature removability, cyclic cleaning, and spray paint tape removal tests. After application and drying, the 2K PU coating with 3% OHSA is clear with a uniform surface and gives even better test results, with excellent signature removability and near-perfect cyclic cleaning results even after four cleaning cycles.
TABLE 2 ǀ Additive performance in a 2K PU coating with a 125 µm wet film thickness.

The long siloxane backbone firmly anchored near the coating surface prevents permanent marker, paint marker, and even solvent-based spray paint from building up sufficient adhesion to the cured coating. Its strongly reduced surface energy causes the paint layers to completely lose adhesion even under minor mechanical loads, such as with the tape release test shown in Figure 5.
FIGURE 5 ǀ Tape release tests of two spray paints applied over 2K PU industrial coatings with 100 µm wet film thickness.

Unlike the previous PUD-based OPV example, the rate-determining step of polyurethane formation under addition reaction has not yet taken place in the 2K PU industrial coating formula. This enables the OHSA to permanently crosslink into the matrix of the 2K PU binder during curing. The hydroxyl content of OHSA, at about 3%, corresponds to common hydroxypolyacrylate binders. Via its OH functionalities, it can add to the isocyanate group of the hardener to form a stable urethane group after its accumulation on the surface, as shown on the right side of Figure 2. In this system, the OHSA additive becomes a highly effective, highly surface-oriented co-binder with long-lasting effects.
This becomes particularly apparent when the OHSA is compared to the standard surface additive, which has a comparatively long polysiloxane backbone but fewer hydroxy-functional anchor groups. The lower hydroxy functionality makes the standard surface additive less compatible with the binder and has less efficiency and permanence in the 2K PU system. In the cyclic cleaning tests, the performance of the 2K PU formula with 3% standard surface additive shows good initial ETC performance that drops by more than half after three cleaning cycles, indicating that the additive itself is being removed from the coating surface with each cycle. However, the 3% OHSA variation shows perfect initial ETC performance that is only reduced slightly after four cycles of isopropanol scrubbing and re-marking.
Conclusions
Polydimethylsiloxane represents the ideal base body for attaching various conventional organic substituents, as well as novel anchor groups. Many suitable structural components can be combined by targeted synthesis according to this building-block approach, creating additives designed for easy-to-clean performance. The high chemical affinity of the anchor groups to the coating binder enables excellent distribution of the ETC additive during the manufacturing process, and provides long-lasting functionalization of the coating surface via chemical bonding or intermolecular interaction. Paint and coating surfaces can be enhanced with permanent effects by using the highly compatible, high-performance ETC additive OHSA.
References
1 Musche, N. Farbe und Lack, 2020, 16-17.
2 Mörk, J.; Pohl, B.; Kottner, N. Richtig. Farbe und Lack, 2019, 18-23.
3 Reidel, E. Anorganische Chemie, 6; Walter de Gruyter, 2004, 204f.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









