Review of Craters in Cathodic Electrodeposition Coatings and their Different Causes

Electro-deposition (ED) has proven its benefit as a unique application technology, resulting in several manufacturers installing ED lines in their paint shops. This is mostly due to a distinguishing feature of this technique, namely the throwing power. This feature allows the coating material to enter into recessed areas of difficult products and bake into corrosion-resistant layers. The key aspect of this application approach is its great transfer efficiency (almost 95%). Normally, the initial paint layer is applied by cathodic electro-deposition (CED) in an automotive paint shop. Air- or high-speed rotary atomizer sprays are used to apply the next layers. Compared to ED, the spraying mode of application has a substantially lower transfer efficiency. This means that using ED to apply either of the next layers would be more cost effective than using a spray gun. Of course, there will be some limitations in terms of color modification. In any case, this technology is currently available for purchase.1
Furthermore, surface morphology is important in the fabrication of super hydrophobic surfaces because the micro/nano structure can trap air and prevent water droplets from spreading when they are deposited on a solid surface. Various surface morphologies, such as square pillar structure, convex structure, pyramid structure, inverted trapezoid structure, re-entrant structure, and over-hanging structure, have been created for producing superhydrophobicity. Due to their low surface energy, long chain fatty acids and silane are frequently employed as modified materials, and they can be absorbed onto the substrate surface.2
Forman and Frank reported that hydrogen gas generated during electrodeposition also causes voids to form on the film, which are not filled during the baking process. These unfilled voids become craters. Additionally, if grease should come in direct contact with the paint or the substrate, a similar effect happens (Figure 1).3

There are many way craters can be form in electrodeposition, including:
- Electrodeposition current (spark discharge);
- Any kind of oil or grease in contact with bath;
- Silicon valves, gasket;
- Bath temperatures, other environmental contamination.
Experimental
Steel Sheet Specimens
The importance of these experiments lies in establishing the alkali resistance of pre-treatments, a property important during deposition, but more important if corrosion begins and alkaline corrosion products come in contact with the pre-treatment galvannealed steel sheets (GA), galvanized steel sheets (GI) and zinc-rich primer-coated steel sheets (ZM) for automotive use. Before electrodeposition coating, the test panels (0.8 x 70 x150 mm) were phosphate-treated by a dip method.4
Materials
The paints used were high-build amino-modified epoxy primers, both standard cure (180 °C bake) and lower cure (-160 °C bake). The substrates included electro- and hot dipped galvanized steel from the U.S. and Europe, and cold rolled steel. They were coated with a variety of zinc phosphate pre-treatments, mainly multi-cation types (Zn-Ni, Zn-Mn-Nil) and all received a chromium-containing final rinse. The main body of work was done with a standard commercial U.S. automotive electro galvanized steel pre-treated with a series of commercial zinc phosphates under carefully controlled conditions and rinsed with a Cr (Ill) final rinse.5
These pre-treatments were:
- Conventional Zn phosphate (high Zn, some Ni modification);
- Zn-Ni phosphate (lower Zn, higher Ni than number 1);
- Zn-Ni phosphate (low Zn, high Nil. Pre-treatment 4 - Zn-Mn-Ni phosphate).
Cationic Electro Paints
An epoxy-based resin and an epoxy-polyamide-based resin were used as cationic electro paints. Both are of the blocked isocyanides kind. The first came from an automobile coating line tank, whereas the second was made in the lab.6
Electrode-Position Coating Method
The electrodeposition equipment seen in Figure 2 was used in this experiment. At a specified temperature, the paint was agitated in the deposition vessel. By providing a specified voltage to one side of the test panel, it was catholically electro painted in the vessel. A SUS 304 stainless steel panel served as the anode. The anode to cathode area ratio was fixed at 2/1. The standard distance between the anode and the cathode was 15 cm. Electrodeposition coating was done at distances of 5 cm, 25 cm and 50 cm in some cases. For the DC power source, a rectifier with a ripple percentage of less than 5% was used. The voltage supplied was varied between 160 and 320 volts. Depending on the applied voltage and anode-cathode distance, the coating thickness was varied using the coulomb static method and ranged from 20 μm to 25 μm. The electrodeposition lasted anywhere from 2 to 5 minutes. After electro coating, the painted panel was rinsed by water.1
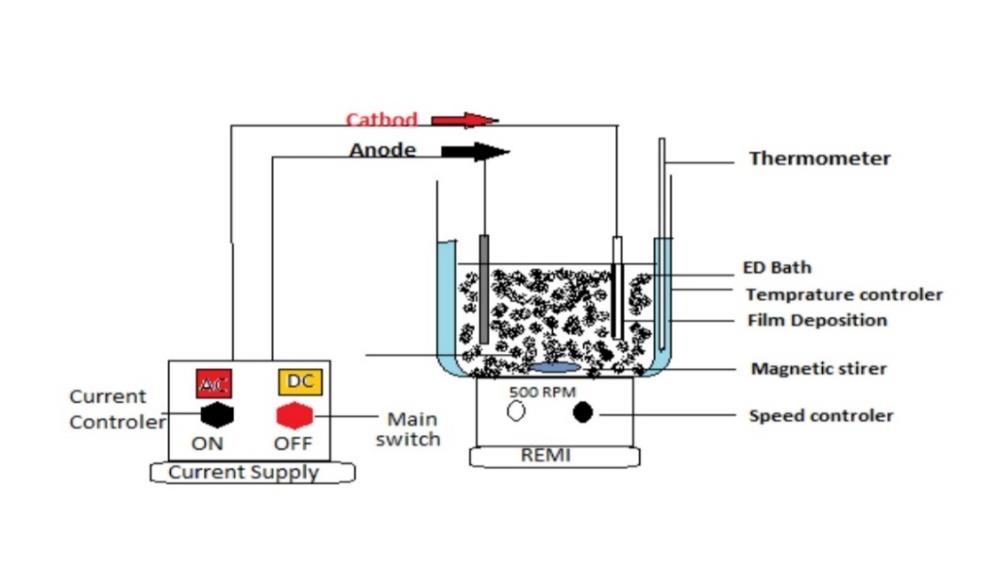


Analysis
Variation of electrodeposition current with time was measured and recorded with an electromagnetic oscillograph. The surface morphology of deposited paint film was observed under a scanning electron microscope (SEM). The softening and flowing behavior of the paint film during the baking process were observed under an optical microscope by heating the deposited paint on a hot plate. Chemical analysis of the baked film was conducted using a grating type infrared spectrophotometer (IR, JASCO IRA-2) and a gel permeation chromatograph (GPC, water: ALC/GPC 244, column: Shodex A803).
Two parts of the film, cratered and non-cratered, were analyzed and their compositional differences were discussed.
In general, electro-paint is dispersed in water, which contains some organic solvent. The organic solvent evaporates gradually by aging. Its concentration in water was measured using a gas chromatograph. The electrical resistance of the tested sheet surface was measured by the DC four-terminal method. SEM absorbed electron beam images of the surface were also observed, which gave an estimation of the uniformity of the surface conductivity.7
Results
Conditions for Crater Formation
Electrodeposition Coating Conditions Have an Impact (Applied Voltage, Anode-Cathode Distance and Bath Temperature)
At a constant bath temperature, a cationic paint produced from an automobile coating plant was electrodeposited on GA at varied voltages and anode-cathode distances. The density of cratering on the surface was also measured. A high voltage and a short anode-cathode distance resulted in a high crater density, as seen in Figure 3. Bath conductivity varies between 1,200 and 1,400 pd/cm. Because of the low conductivity, the IR drop is quite high. As a result, the anode-cathode distance can be thought of as having the same effect on crater density as the applied voltage.6
Electrodeposition coating was also done on GA with a consistent anode-cathode spacing at various voltages and bath temperatures. As demonstrated in Figure 4, the voltage required for crater formation reduced as the bath temperature increased. The relationship of bath conductivity on temperature, as illustrated in Figure 4, explains this.
To summarize the findings, Figures 5 and 6 below show measures that are effective in reducing cratering in electrodeposition coating.6


Zinc Phosphate Pretreatment
During deposition, pre-treatment disintegration occurs, although the specific implications on performance are unknown. Another interaction, pin holing/rupture, has a negative impact on aesthetics and corrosion resistance, which is understandable given that the flaws are holes or thin patches in the primer. The consequences of pre-treatment disintegration should be milder. The impact of dissolution on corrosion resistance will be discussed later in this article, although further research in this area is needed. Unpublished field and proving ground findings, on the other hand, demonstrate no discernible corrosion differences between coatings with low and moderate dissolving tendencies. Despite some breakdown of one of its components (pre-treatment) and probably a smaller amount of dissolution of another, it appears that the overall system is functioning well (zinc-coated steel). For the ED primer to anchor through the pre-treatment to the substrate beneath, dissolution is required. The anodic treatment also resulted in a significant increase in the porosity of the zinc phosphate. Porosity should rise with cathodic deposition, although this isn't clear, and we haven't measured any changes yet. The alteration of zinc phosphate by the soluble lead in the ED coating is a highly beneficial interaction between the ED bath and pre-treatment. Pb ions are integrated into the phosphate crystal during the deposition process. It's possible that Pb(P04) and Ze Fe Pb (PO,) are produced. The phosphate layer becomes more alkali resistant, limiting corrosion's spread and reducing the damage it causes.5
Dependence of Crater Density on Steel Sheet and Paint
Figure 7 depicts the link between crater density and the voltage at which cratering begins. Crater density varies based on the type of steel sheet utilized. Epoxy polyamide-based paint, on the other hand, has a similar cratering propensity. There were no craters on any type of steel sheet when applied at low voltages. Cratering began on GA (at 220 V), then moved to ZM (at 250 V) and lastly to GI when the voltage was raised (at 280 V). Craters appeared only when CR was subjected to voltages greater than 300 V.8

Effect of Paint Aging (Behavior of Organic Solvents)
Various organic solvents are added to a cationic electro-paint to change the dispersibility and film levelling capabilities of the resin.
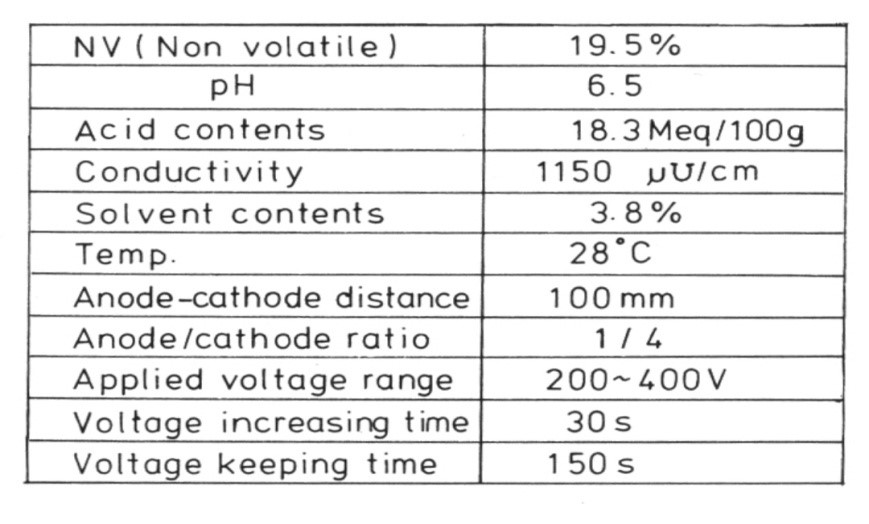
Table 1 shows the solvent content of a paint used in an automobile coating formulation. Ethylene glycol monoethers (cellosolves), carbitols and ketones are the main solvents. MIBK is used to modulate viscosity during resin synthesis and paint preparation. After the paint bath has been created, it is gradually evaporated. The effect of the solvent content of the paint on cratering was investigated. The original paint was used to create a cationic electro-paint in the lab. It was then matured in an open jar at room temperature.
Solvent |
Content (Vol%) |
1. Miscellaneous |
0.39 |
2. Ethyl cellosolve |
3.12 |
3. Butyl cellosolve |
2.16 |
4. Carbitol |
1.01 |
5. MIBK |
0.08 |
TABLE 1 ǀ Solvent content of the cationic electro-paint.
During the aging process, GA was electro-coated at predefined intervals, and the variation in solvent content was measured using a gas chromatograph. Figures 8 and 9 depict the results. The coating thickness and cratering behavior altered as the aging duration increased. Shortly after the bath preparation, the paint layer was deposited up to thicknesses more than the allowed value (25 pm) without causing craters. As time went on, craters began to emerge. The paint thickness had fallen to the target value of 25 mic after 20-25 hours. As seen in Figure 9, MIBK levels decreased with age and were scarcely detectable after 24 hours. When the MIBK evaporated, the coating thickness reached the predetermined value, and cratering occurred. As a result, the MIBK is most likely due to cratering.1


Hydrogen Evolution Mechanism and Oxygen Absorption Mechanism
The creation of anodic and cathodic areas through which current travels through the conducting solution causes corrosion. The anodic reaction involves the breakdown of metal as equivalent metallic ions and the liberation of free electrons. The anodic reaction consumes electrons with either M Mn+ + ne-(oxidation), or the cathodic reaction consumes electrons with either M Mn+ + ne-(oxidation).
Depending on the nature of the corrosive environment, (1) hydrogen evolution or (2) oxygen absorption may occur. When two distinct metals come into contact with a common conducting liquid (medium), the metal with the larger oxidation potential functions as an anode, and corrosion occurs. The cathode is a second metal with a lower oxidation potential, and it is this metal that is protected (i.e. the anode corrodes while the cathode is protected.) Galvanic cell corrosion is another name for this. This sort of electrochemical corrosion manifests itself in two ways: (1) with evolution of hydrogen; and (2) with absorption of oxygen.
Evolution of Hydrogen
Electrochemical corrosion with evolution of H2 occurs in acidic environments:
At anode Fe →Fe2+ + 2e-(oxidation /corrosion)
At cathode 2H+ + + 2e-→H2
Overall reaction Fe +2H+ →Fe
Explanation: (1) Iron tank, which acts as an anode undergoes corrosion as Fe atoms from the tank pass into the acidic solution as Fe++ ions, as shown in reaction above. Since nothing but loss of Fe atom as Fe++ ions, i.e. corrosion. (2) Free electrons accumulate at cathode. Hydrogen ions present in acidic solution take up these electrons, forming H2 gas as shown in the reaction above. Also refer to Figure 10. Ed bath H2 gas liberates in the form of bubbles near the cathode. When the film is deposited it ruptures the film and craters will form. Thus, hydrogen evolution type of corrosion is nothing but displacement of H+ from acidic solution by metal ions. All the metals above H2 in the electro chemical series get dissolved in acidic solution with simultaneous evolution of hydrogen. In hydrogen evolution type of corrosion, the anode has a large area (like metallic tank) and the cathode has a smaller area.2
Absorption of Oxygen
If electrolytes are neutral or in an alkaline aqueous solution, corrosion takes place by absorption of O2 - rusting of iron in water containing dissolved oxygen occurs by oxygen absorption mechanism. At anodic area iron will dissolve by oxidation. Figure 11 shows the surface of iron is usually coated with the thin film of iron oxide. But if this iron oxide film develops some cracks, anodic areas are created on the surface while the metal (iron) act as cathodes. Here the anodic areas are small surface while the rest of the surface of the metal forms large cathodes. At anode, Fe →Fe2+→2e-.[9]


Evaluation of Electrodeposition Coating Conditions and Lacquer Ring Resistance
The pressure gauge used was Yokogawa Hokushin 2011-40, and the current meter is Yokogawa Hokushin 2011.The accuracy was 0-5% in all cases. We used the Yokogawa Hokushin 3056 pen formula.8
|
Chemical Composition |
Zn 2+ |
800 ppm |
Ni2+ |
800 ppm |
|
Mn2+ |
600 ppm |
|
F- |
500 ppm |
|
Temp |
43 °C |
|
Time |
120 seconds |
|
TABLE 2 ǀ Conditions for zinc phosphating.
Energization Requirements
(1) Transmission start: Boosts at a constant speed and reaches the specified voltage at 30 seconds.
Boost with:
(2) Constant voltage electrodeposition: After boosting, electrodeposition coating with 150 seconds specified voltage
After electrodeposition, wash the surface of the coating film with water under the condition of 1800 C x 20 minutes.
(3) Dried: Observing the surface of the coating film, craters have not developed. Count the number of items and convert them to the number equivalent to dm2 bottom.6
Results and Consideration
The surface condition of the used steel plate, and the steel plate. Above, Table.3. the SEM copy of the phosphate skin film formed under the above conditions, Shown in Figures 12 and Figure 13 of each. Zinc salt skin crystals shown in Figure 12 are Fe / Zn and Figure 13 phosphating on CR Conclusion Fll. = Zn2Fe (PO4) 2.4H20
And, Hopite conclusions on EG and GA} 1 [II [: Zn: 3 (PO4) 2 It is classified as 4H20. Here, C/W is the unit area. The amount of crystal deviation of the skin membrane is 1 and P (%) is the X-ray of the crystals. Phosphophy1-expressed by Eq. (1) From the DLL data this is the crystal formation ratio of Light.10


Infrared Spectrographic Analysis (IR)
By the methods shown in Figure 14, the nuclei were isolated from the paint and examined using IR and GPC. Figure 15 displays the nucleus' IR spectra on GA. The nucleus features an absorption band with a wave number of 1,388 cm-1 that does not exist in either the initial paint liquid or the usually deposited paint's IR spectra. It exhibits a strong band (2,960, 2,920, and 2,848 cm-') for aliphatic CH2= and CH3 stretches.1


Gel Permeation Chromatography (GPC)
The nucleus has a higher molecular weight than the ordinarily deposited layer, according to GPC tests. The hatching portion of Figure 16 corresponds to the rise in molecular weight. The increase of epoxy resin is seen by the peak near THE-25 mL. Using a calibration curve for diglycidyl ether of bisphenol A (DGEBA) epoxy resin, the increase was determined to be around 3,000. The molecular weight of regular paint was estimated to be over 900.1

Behavior of the Paint During Baking
For the paint deposited on GA and CR, the softening and flowing process was noticed during baking. When the temperature hit 60 °C, the paint began to soften and flow, indicating that the levelling process had begun. The levelling around the nucleus on GA was not as smooth as it should have been. Small nuclei were gradually covered with paint, but large nuclei remained exposed until the curing reaction was complete. They repelled the flow of paint in the area. Craters were produced as a result. The surface of the paint film on GA changes during baking. On the other side, on CR, the levelling was accomplished quickly and there was no cratering. It was also discovered that when the paint film was fused, the H2 gas contained in the film escaped and did not form a crater.1
Electrical Resistance
The DC four-terminal approach was used to test the electrical resistance of the surface of GA, GI, CR and ZM. Table 4 shows the results. The maximum resistance was found in ZM, which possesses an organic film. In the order of GA, CR and GI, resistance reduced. The surfaces of GA, CR and GI are seen in absorbed electron beam images in Figure 17. The absorbed electron beam is strong in many white places in GA. Non-uniform electrical properties are the result of this. The non-uniform absorption was not visible in the GI or CR. According to the X-ray diffraction investigation presented in Figure 18, GA contains a Zn-Fe alloy coating layer. The Zn-Fe layer is made up of three phases: 1, vj and P. Electroplating was used to prepare these Zn-Fe alloy phases, and the electrical resistances were measured using the same procedure. For the measurements, a columnar electrode with a diameter of 3 mm was employed. Under a 10 kg electrode force, the following values were obtained: 1 layer: 0.43 mfl, P layer: 0.53 mfl, and r layer: 0.08 mS2. The non-uniform surface conductivity appears to be caused by the non-uniform composition of the Zn-Fe layer.7
Type of Steel Sheet |
GI |
CR |
GA |
ZM |
|
|
Electrode force Dia. 3 mm |
10 kg |
0.033 |
0.224 |
0.224 |
7.18 |
20 kg |
0.024 |
0.077 |
0.200 |
4.72 |
|
TABLE 4 ǀ Electrical resistance of steel surface.


Discussion
Cratering Process
From the experimental results, the cratering process in the cathodic electrodeposition coating is summarized as follows:
(1) H2 gas generation and paint deposition occur shortly after electrolysis begins, and the porous paint film and gas layer are applied to the steel sheet cathode. Spark discharge occurs locally on the steel surface when electrodeposition is done at a high voltage.
(2) The deposited paint near the discharge point undergoes a thermal reaction as a result of the spark discharge. A portion of the paint transforms into nuclei with properties that differ from those of typically deposited film.
(3) During the flow process of baking, these nuclei do not easily fuse into the paint layer. Large nuclei obstruct the flow of paint, resulting in the development of craters. This procedure is depicted schematically in Figure 19.1

Measures to Prevent Cratering
Cratering can be avoided if the spark discharge during electrodeposition is suppressed. The following measures are effective in this regard.
1.) Make the surface conductivity uniform to prevent local current concentration. Controlling the galvanizing and annealing processes helps make the alloy layer homogenous for GA. Allowing good conductivity r phase to spread consistently on the top layer of coating or covering the surface with a uniform coating of Fe, Zn, or other metal is also effective.5
2) Release the generated H2 gas from the cathode surface easily by adjusting the surface tension of paint, strengthening the stirring in the vessel and vibrating the steel sheet to be coated.10
3) Reduce the applied voltage.
4) Prepare a cationic electro-paint that ensures a low electrical resistance of the deposited paint film. An electro-paint containing large amounts of low boiling point solvents does not cause cratering. This is because the resistance of the deposited paint film is low. A paint designed to produce a thick deposited film of low resistance would be very effective in preventing cratering.1
Conclusions
The cratering in cathodic electrodeposition coating was investigated and the following results were obtained:
- Cratering happens when a portion of the paint suffers a temperature change as a result of the cathode's spark discharge. Nuclei are formed, which prevent the applied paint coat from levelling.
- Cratering is more common on GA and ZM than GI and CR. GA has a high crater density due to its non-uniform surface conductivity.
- The process of forming the crater is during electrodeposition coating. Hydrogen generated locally and violently in the analyzed coating film it was destroyed by the gas and became a void-like defect.
The above defects are concealed by heat flow even during the thermosetting process. It becomes a crater without being lost.
- By energizing the slope that simulates the motor vehicle line when electrodeposition coating was applied, no spark discharge occurred, and therefore, the spark discharge causes cratering in a blink of an eye. It is considered to be limited to the case of electricity.
- Zn-Mn-Ni phosphate pretreatment are more resistant to attack during electrodeposition than
- conventional phosphates (which usually have some nickel modification). This greater resistance is due to the lower alkali solubility of Zn-Mn-Ni phosphate.
- to prevent spark discharge during electrodeposition, take the following precautions:
- Make the steel sheet's surface conductivity consistent.
- Prepare a cationic electro-paint that results in a low-electric-resistance deposited paint coating.
- The applied voltage is reduced.
- This makes it easy for produced H2 gas to escape the cathode surface.
References
1 Cratering Galvannealed in Electrodeposition Steel Sheet * Coating of Cationic Paint on By Minoru KITAYAMA ,** Tadao AZAMI ,** Nobutaka MI URA *** and Tadashi OGASAWARA **, (1984) 742–750.
2 T. Kubota, M. Yamashita, カチオン電着塗装におけるクレータリング発生機構, 鉄と鋼. 77 (1991) 1087–1094.
3 4m mmmmmmvm, 2018.
4 S.H. Chen, T. Takasugi, D.P. Pope, The effects of trace impurities on the ductility of a Cr-Mo-V steel at elevated temperatures, Metall. Trans. A. 14 (1983) 571–580. https://doi.org/10.1007/BF02643774.
5 C.K. Schoff, The effect of the electrodeposition process on pretreatments, SAE Tech. Pap. (1991). https://doi.org/10.4271/912297.
6 R.G. Hart, H.E. Townsend, Mechanism of cathodic electrocoat primer cratering, SAE Tech. Pap. (1983). https://doi.org/10.4271/831818.
7 L. Gitleman, 済無No Title No Title No Title, Pap. Knowl. . Towar. a Media Hist. Doc. (2014).
8 N. Sato, Noboru SATO, (1986) 88–93.
9 R. Papoular, R.B. Zipin, Electrical Phenomena in Gases, Am. J. Phys. 35 (1967) 363–363. https://doi.org/10.1119/1.1974097.
10 강용묵, 리튬 이차 전지용 음극소재 개발No Title, (n.d.).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








