Farm to Lab Sustainable Silicone Coatings Additives

Over my 33 year career (so far) in organomodified silicones, customers have occasionally asked me for green, natural or sustainable products. The early requests were infrequent, usually for very specific circumstances, and somewhat vague with the askers often uncertain what their choice of terminology meant. Recently, these requests are coming more frequently and the queriers clearly want products that are not based on petroleum. The origins for these are the beliefs that petroleum is non-sustainable long term and that a fossil fuel economy is harmful to the Earth we all call home.
In the last two years it seems the “Covidity of Errors” that we have lived through has dramatically reinforced this market driver. Whether it was facing one’s mortality or seeing first-hand how inadequately large-scale global problems are dealt with by society, the pandemic has turned this market driver into a market need. While there are likely other factors that have “driven this driver”, today’s reality is that people want products made from botanical plants instead of petroleum plants.
There are other more complex parallel considerations such as total carbon footprint that look at a more complete picture of a material’s impact on the environment[i]. These are likely more effective but Human nature is to look for a short-cut and biological plant-based is an easy identifier.
Coatings additives provide foam control, rheology modification, enhanced surface properties and/or improved appearance to coating films. There are differing types of chemical additives that provide this performance. This paper focuses on organomodified silicones, which represent a large share of the types used to provide flow and leveling, wetting, slip, release and protection properties. These additives are ubiquitous in small quantities – usually fractions of a percent – in industrial coatings.
So how sustainable are these silicone materials? Before talking specifically about organomodified coatings additives, let’s go on a brief tangent about the background of the base silicone polymer. Silicone is a man-made polymer invented in the petroleum boom and is known by a few different names. Polydimethylsiloxane, often abbreviated as PDMS, is a common and arguably more correct synonym for silicone. This is often shortened to siloxane[ii]. In cosmetics, silicone is offered under the INCI names dimethicone, dimethiconol and cyclomethicone[iii]. Dimethicone, along with its derivatives, are very frequently used in skin and hair products at multiple percent levels.
In pharmaceuticals, Simethicone is an FDA-approved drug for treating digestive gas and bloating[iv]. Defined in the U.S. Pharmacopeia as 93-96% silicone oil and 4-7% hydrophobic silica, this same structure is used to control foam in many industrial and food grade applications. Of course Simethicone is made under USP standards, but chemically it is the same as industrial silicone antifoam products.
As you might infer from above, these materials are generally regarded as safe and used in many consumer products. After some of the major manufacturers were litigated over the safety of breast implants in the nineties[v], the industry proactively combined resources and conducted a tremendous amount of toxicity testing[vi]. Very little toxicity was found, and much of what was seen is believed to be specific to the animals on which the testing was done.
It is interesting when I talk to people that everyone knows the FDA stopped the use of silicones for implants, but few people realize the FDA allowed them again after the facts were known[vii]. Bad news is more newsworthy. Today, silicones are used extensively in medical devices due to their low toxicity and low rejection by the body’s natural defenses.
But are they made from petroleum? Yes and no. Silicones are by weight mostly Si and O mined from the earth, but silicone polymers are partially petroleum derived. Nearly all of the silicone manufactured today is made from the reaction of elemental silicon metal with single carbon atom petroleum feedstocks under rare earth metal catalysis and high energy conditions. The petroleum feedstock is derived from methanol, which is in turn derived from natural gas. It is possible to make methanol in a non-petroleum green process, but that is not the predominate process today[viii].

Is this sustainable? Silicon is the second most common element in the earth’s crust behind oxygen. Found in the 1:2 ratio of silicon dioxide, the two elements comprise nearly 75% of the terrestrial elements[ix]. While most of the silicon in the planet is tied up as silicon dioxide in a multitude of geological forms, silicon is manufactured in large quantities for many industries including solar cells, electronics and silicones.
So why use an “unnatural” polymer at all? A fair question with a straightforward answer, silicone behaves very uniquely. Relative to organic polymers, the silicon atoms stabilize the polymer to free-radical decomposition pathways such as oxidation and thermal breakdown. This leads to uses in high-temperature applications such as outdoor grills and car exhausts, as well as harsh environments such as space. Also, the polymer is thermally and electrically insulative, allowing unique performance in electronic encapsulants.
The bulk surface tension of silicone oil is 20 mN/m. Pure water is 72 mN/m and most organic polymers are in the 30-45 mN/m range. Only fluorocarbons provide surface tensions lower than silicone with values in the mid-teens (mN/m). This low surface tension as well as low interfacial tensions manifests in wetting, flow and leveling, COF reduction, slip, surface protection, pencil hardness, interfacial stabilization, desirable haptics and other properties that are hard to achieve with anything else. Fluorocarbons provide some of these properties but seem to have far more regulatory and sustainability concerns.
As we know in life, there is always a “but”. BUT, silicone is not soluble in water or organic oils. It forms a separate phase, and while that enhances its activity in some surface applications, most of the time that is a problem. In coatings additives, this separate phase is great for a hammer tone finish in powder coatings, but bad for everything else. Silicone itself has a bad reputation in paint shops due to its power to cause defects. The incompatibility and low surface tension are the causes of this.
To create coatings additives we had to solve that problem. It’s true that emulsification into water or using other materials to compatibilize the silicone sometimes works, but the only certain way is to chemically modify the silicone with organic groups. These organomodified silicones own the lion’s share of silicone-based coatings additives used today.
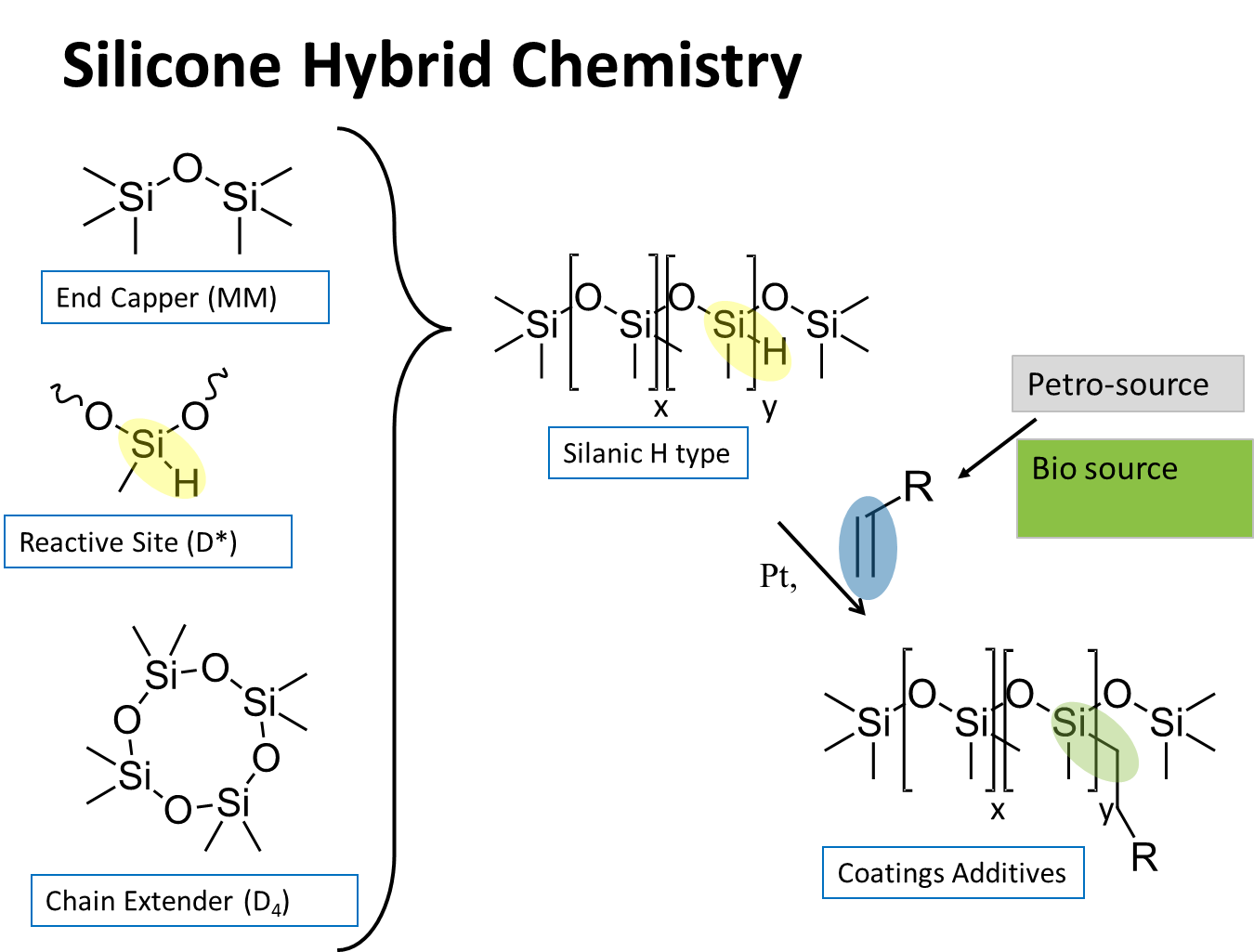
Alpha-olefins and allyl started polyalkyleneoxides are the main feedstocks used in the industry to make these coatings additives. Today, these organic polymers are manufactured from petrochemicals. Ultimately, ethylene is used to make both of these common organic modifiers[x].
Recently, a source has come onto the market for the polyalkyleneoxides that are ultimately derived via ethylene from sugar or corn ethanol. The bio-ethylene is converted into ethylene oxide and plugged into the existing petroleum procedures. The allyl polyethyleneoxide materials are very similar to the corresponding petroleum-based materials that we buy today. Minor differences in dispersity and average chain length are within a reasonable range.
The polyethyleneoxide-modified silicone materials are a very common coatings additive type. While still insoluble in hydrocarbon oils, any heteroatom-containing material such as commonly used esters, glycols, ketones, etc. is sufficiently polar to be compatible with these polyether silicones. Also, they have the advantage of conferring compatibility into water-based systems. The oxygen atoms provide sites for hydrogen bonding of water pulling the polymer into solution.
What follows is a summary of Siltech’s approaches and options for bio-sourced organic materials reacted with silicone to create coatings additives.

Our first bio-sourced organomodified silicone was our Silubeâ CO castor oil derivatives. Intended as more natural ingredients for personal care, one of the early market segments asking for natural products, these were transesterified from the triglyceride castor oil. We did briefly evaluate these products in coatings, but they did not show promise and we left them for PC. Silube CO Di-45 exemplifies the best of these, showing good COF reduction and slip but poor flow and leveling. We did not look at an exhaustive series of these as there was no market driver then but the few we examined all exhibited poor flow and leveling.
Another bio-sourced organosilicone material that has been seen in the market is Eugenol modified silicones. These are currently sold in niche markets but not in coatings. Made from the hydrosilation of Eugenol, an essential oil isolated from cloves, these products have a more interesting structure for coatings additives. Similar aryl derivatives made from methyl styrene, such as our Siltech 3H-12MS, are used quite effectively as coatings additives. Siltech has not yet evaluated these Eugenol derivatives in coatings. Even though they may perform well, cloves are a less practical bio-source than corn or sugar cane.
Most recently, we have made a representative grid of some of our standard coating additives from this newly available bio-sourced polyethyleneoxide and compared those to the petroleum-sourced analogues. We utilized a variety of comparisons such as foaming, surface tension, wetting, COF and leveling. The goal was to determine if they behaved the same within lot-to-lot variability.
The seven products we synthesized include two excellent wetting agents, two strong slip agents, one “middle of the road” additive good for wetting and slip, and two linear structures. These last two are known to be the best for slip, protection, anti-graffiti and similar applications due to the efficiency with which they orient at an interface. These seven range from 30-80% bio-organic content with the remainder being the silicone polymer. We have confirmed the biological origin with outside testing that uses radioisotopes to determine the petroleum content.
For simplicity, and as the well-known structure property behavior is not the point of this white paper, these are labeled A-G with -P or -B for petroleum or bio-sourced respectively. In some of the evaluations some sample pairs were not soluble enough for testing.
Here are the results for some basic properties. Column three shows our mean/standard deviation as well as specification for the corresponding commercial (-P) products.

While there is a significant bias towards higher viscosity and different surface tension reduction, we believe this is due to the slightly different polydispersity profiles and average MW of the polyether samples. It is worth noting that only the B-B sample is outside of our specifications.
Likewise aqueous foam heights of 1% solutions, measured with a graduated cylinder and nitrogen sparge, show little differences.

Of course, it is not enough to go by physical properties. We compared the performances of these pairs of products in a WB PUD and a UV-cured acrylate resin formulation. Appearance, foam, flow and leveling were all the same within a pair.
COF is a convenient and often discerning property. Below are the COF data in the PUD and then the UV system. Kinetic COF is the more relevant since overcoming the tackiness of the sled/coating interface can cause a false interpretation of the static COF.

Finally the COF data for the UV cured system is identical

The availability of the bio-sourced polyethyleneoxide polymers suitable for making organomodified silicone coatings additives is exciting, and we think these Silsurfâ bio products can be quickly adopted by anyone wanting to improve their sustainability. However there are still a few limitations.
Our silicone polyether bio-sourced options today are limited to EO chain derivatized materials. The polypropyleneoxide derivatives are not available from biological sources. This is a limitation that in most cases, will be readily surmountable. However, there is a small group of polypropyleneoxide-modified silicones that are among the best products known for foam control in WB coatings. For these it will be hard to match the performance without a PO bio-source.
The most important use of PO in these chains is to lower the crystallinity and T(g) of the materials by using EO/PO modified silicones. The pour points of PO containing silicone polyether derivatives are normally well below the temperature extremes seen in shipping, but the EO exclusively chained materials have pour points that can be reached during cold weather shipping. The effects of this are usually completely reversible upon warming.
Another key point of EO/PO silicone polyether additives is that they are GRAS listed by CAS number under an indirect food contact regulation[xi] and so are often easier to gain food-grade compliance. The underlying low toxicity and other considerations used in food-grade determinations would apply to the all EO materials as well so they could be, and in some cases have been, granted food compliance by the FDA.
In other words, there is still a lot of work to be done in this area. We at Siltech look forward to doing it.
[i] https://css.umich.edu/factsheets/carbon-footprint-factsheet for one of many references on this evolving approach.
[ii] https://en.wikipedia.org/wiki/Silicone Offered in lieu of delving into a tangent within a tangent with silicone pseudonyms, this Wikipedia summary page is a good start for silicone and synonyms.
[vi] https://www.americanchemistry.com/industry-groups/silicones-environmental-health-and-safety-center-sehsc
[viii] https://www.methanol.org/renewable/ and many other references available
[xi] C.F.R 176.210 which governs the components of defoamer formulations used in pulp and paper which is used to make food containers lists CAS# 71965-38-3 as generally regarded as safe.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









