Degradation of Automotive Clearcoat Caused by Bird Droppings

The need for automotive coatings to have a proper appearance and aesthetic properties has led to a vast amount of research focused on the study of their weathering performance.1 In this regard, humidity and sunlight are two factors that have been thoroughly investigated. Other environmental factors, such as those that originate from biological sources, have not been investigated systemically. Various biological substances such as bird droppings, tree gums and insect gums can have an impact on the appearance of a car body during its service life. Apart from the lack of knowledge of such a phenomenon with respect to the short-term or long-term effects, the current standard test methods for evaluating resistance to these substances are not sufficient and cannot properly cover all ranges of biological attacks. In general, Arabic gum is considered a universal simulated chemical to study the biological resistance of automotive coatings. This, however, may not represent all biological materials to which coatings are exposed in real-world conditions.2
This article is part of a comprehensive work aimed at studying and characterizing coating degradation caused by various biological substances. Typical defects observed on an automotive clearcoat exposed to bird droppings and pancreatin (the synthetic equivalent) were recorded by a digital camera and are shown in Fig. 1.11
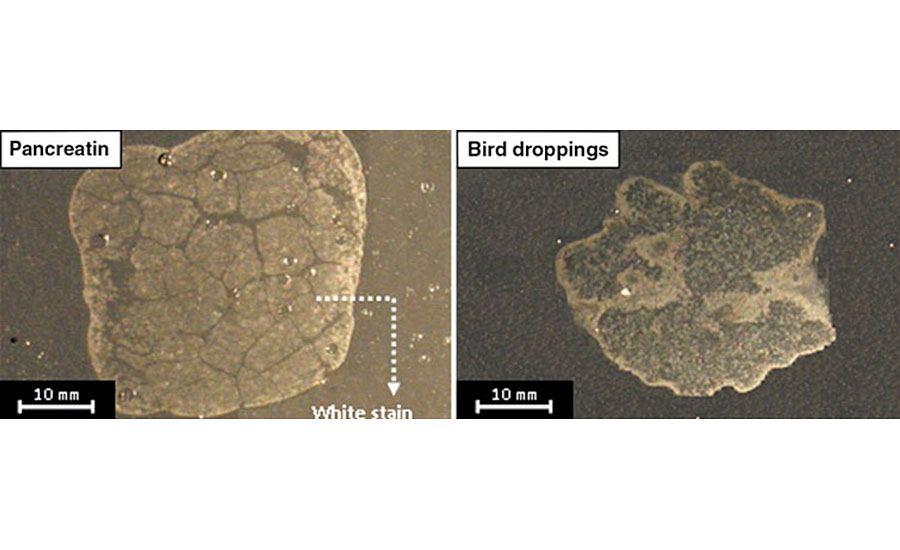
These defects appear as stain-like white areas. The edges seem brighter and more affected compared with the interior areas. It was found that bird droppings decrease the appearance parameters of the clearcoat, i.e., gloss, distinctness of image and color values, therefore affecting the aesthetic properties of the coating system. Thermal-mechanical studies also showed that hardness, glass transition temperature and crosslinking density of degraded clearcoats decrease in the presence of bird droppings.3 Also, the influence of aging method (pre-aging or post-aging) and the chemical structure of clearcoats against such bio attacks were reported. It was observed that the post-aging process, which simultaneously exposes bird droppings and UV radiation to coatings, degrades the clearcoat much more intensively than pre-aging, in which only bird droppings on pre-weathered clearcoats are exposed. The investigation of clearcoat chemistry revealed that incorporating higher ratios of melamine crosslinker, in spite of this resulting in a higher crosslinking density, may lead to an inferior biological resistance.
It was also found that although the main process was a hydrolytic cleavage, the mechanism and the influencing factors to pursue this hydrolysis reaction have remained unknown. In this study, we intend to reveal the cause and the mechanism of coating degradation upon exposure to bird droppings. Different possible hypotheses are presented, and the reasons to accept or confirm each one will be been discussed.
Due to the unknown ingredients of bird droppings and the difficulty in collecting sufficient samples, we used pancreatin to evaluate its ability to simulate bird droppings.
Experimental
Coating Preparation
The coatings used in this study were a multilayer automotive system (Figure 2) composed of an epoxy amine electrodeposited layer, a polyester melamine primer and a black acrylic melamine basecoat applied on phosphated steel panels. The final layer was a typical acrylic melamine clearcoat applied through a wet-on-wet method. The curing process of basecoat/clearcoat was done simultaneously at 140 ˚C for 20 min.

Biological Materials
The two biological substances utilized were natural bird droppings and the synthetic equivalent, pancreatin. The natural bird droppings were collected from a sparrow bird, and the pancreatin was purchased from Merck and used as received. Slurries of both were prepared (10:1 water: biological materials).
Biological Tests
Since, in most cases, a coating is exposed to both climatic and biological substances simultaneously, these conditions were simulated in the laboratory. After curing the coating, a slurry of bird droppings and pancreatin was placed on the coating, and the sample was transferred to a weatherometer. Samples were placed for 300 hrs in a programmed weatherometer according to PSA D27 1389. Some specifications of the weathering conditions are as follows. Internal and external quartz filters, continuous 0.4 W/m2 radiation with a wavelength of 340 nm, no water spraying and temperature at 60 ± 3 °C. To keep the samples wet, they were removed every 100 hrs to be sprayed with water.
After completion of 300 hrs exposure, samples were removed from the weatherometer, wiped and washed several times to remove all unattached biological substances. After completely removing the biological materials, the coating surfaces were evaluated by macroscopic and microscopic techniques. Since it can be expected that the weathering and biological materials may have a synergistic effect, separation of such effects helps us to effectively follow the biological degradation mechanism of the coating. To this end, two samples that were only subjected to 0 and 300 hrs weathering (without any exposure to biological substances) were used as references.
Characterization
In order to follow the chemical variations of clearcoats caused by weathering and/or biological attack, FTIR spectroscopy was carried out. A Bruker FTIR spectrometer Model IFS48 was utilized. Samples used for FTIR analysis were removed from the clearcoat surface and mixed with KBr powder to prepare pellets. The optical image and topology of the degraded surface after the biological test were recorded by the aid of a Leica DMR optical microscope and a DME Scanner atomic force microscope (AFM) DS 95-50, respectively. 1 cm² of each sample was utilized for AFM analysis. Also, to further characterize the degraded area, a Sungwoo Corp. scanning electron microscope (SEM) equipped with an energy-dispersive spectrophotometer (EDS) analyzer was utilized. A sputtered layer composed of Au and Ti was deposited on samples. For characterization of bird droppings and pancreatin, X-ray fluorescence (XRF) spectroscopy was employed. The pH of the biological material slurries was measured by a HANNA PH 211 pH meter.
Results and Discussion
Microscopic Evaluations
In real-world conditions, a clearcoat almost always experiences both biological and weathering (sunlight and humidity) conditions. As described in the experimental section, such conditions were simulated on a typical acrylic melamine clearcoat. Figure 3 shows the optical and AFM images of the clearcoat surfaces before and after being exposed to various conditions. These included 300 hrs weathering and/or biological attack. This figure shows that the surface of the clearcoat before any exposure is smooth. However, both weathering and biological attacks have roughened the surface. Also, it is evident that this roughening phenomenon becomes more severe when both weathering and biological conditions are introduced simultaneously. Many sharp points can be seen on the clearcoat surface that has only been exposed to 300 hrs weathering. However, in the clearcoat exposed to simultaneous weathering and biological materials, some blunt areas can be observed, along with various pits and valleys. These micro- and nano-scale features are believed to be an indication of an etching phenomenon caused by biological and weathering conditions. With respect to the features of samples after exposure to 300 hrs weathering, or even those exposed longer in weathering (i.e., 1,000 hrs), and from the results of various studies for the clearcoats only exposed to biological substances, it can be shown that the role of biological materials in causing such effects is much greater compared with that of weathering.
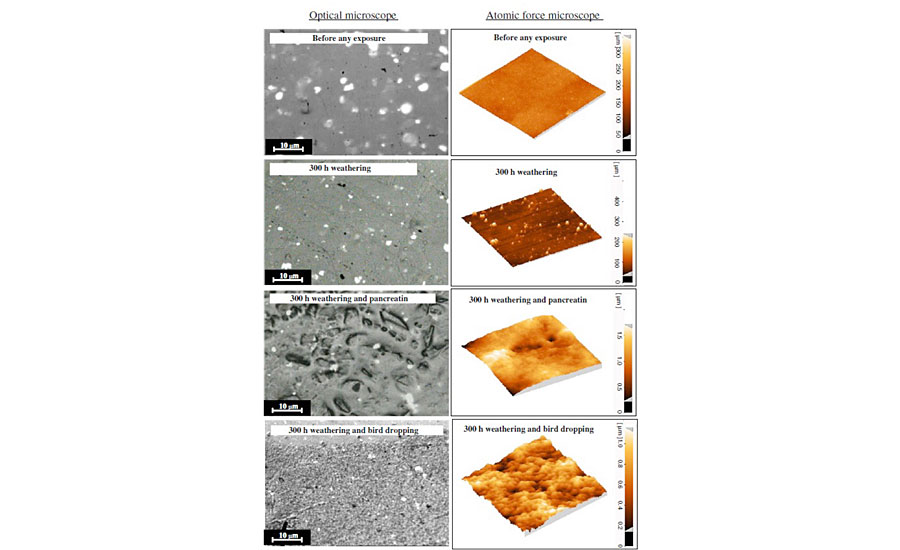
As stated before, pancreatin as a synthetic substance was used in this study to simulate the biological attack of bird droppings. The comparison of optical microscopic images obtained by exposing coatings to pancreatin and bird droppings illustrates that, although both have been etched, the resulting features are, to some extent, different. While pancreatin creates interrupted and deep-etched areas, bird droppings produce shallower roughness. AFM images clearly demonstrate the differences between pancreatin and bird droppings at the nano-scale.
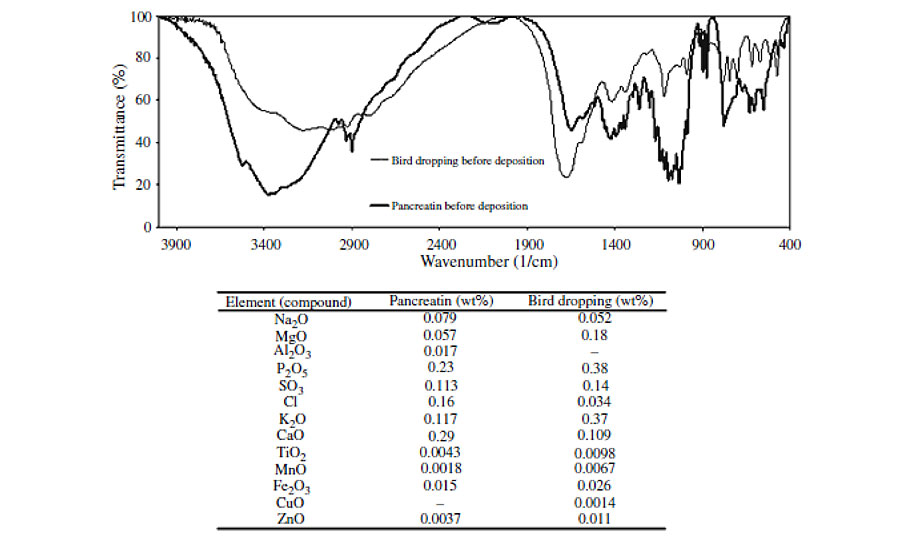
In order to study the variations in chemical structure of clearcoats after exposure to weathering and/or biological substances, FTIR spectroscopy was utilized. Figure 4 shows FTIR spectra of clearcoats before and after being exposed to various conditions.
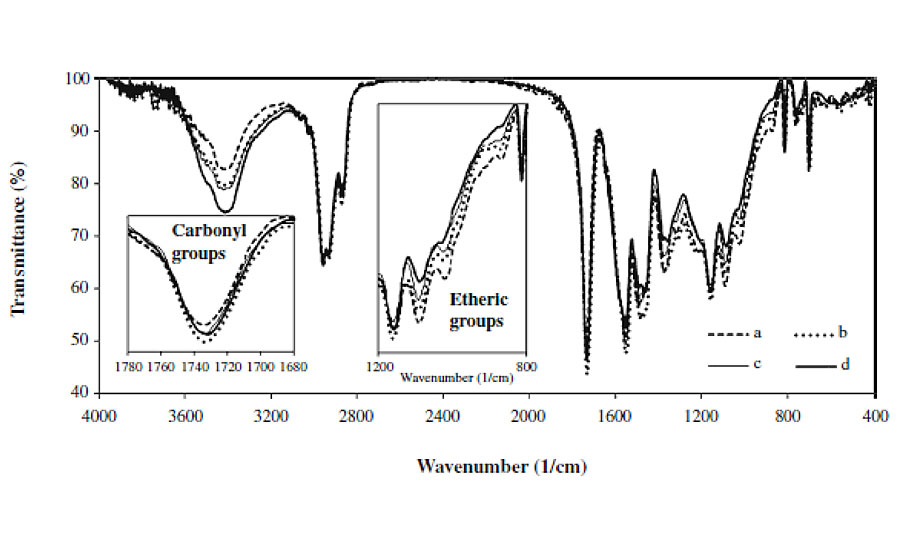
All spectra were normalized by taking the CH vibration peak around 2600-3000 cm-1 as an internal reference. The FTIR spectra show that the intensities of the peaks corresponding to various species of carbonyl groups (around 1,730 and 1,680 cm-1), hydroxy and amine groups (around 3,100-3,600 cm-1) increase. Meanwhile, it is found that the intensity of peaks corresponding to various species of etheric bonds (around 900-1,100 cm-1), acyclic (at 1,350 cm-1) and cyclic methylene bridges (at 1,480 cm-1) decreases. These variations are related to photo and hydrolytic reactions of automotive coating.6, 8
During the hydrolytic reactions of acrylic melamine coatings, etheric bonds are broken, resulting in different species of NH and OH, i.e., methylol melamine groups. These generated groups may self-condense with each other, forming melamine–melamine linkages. Also, there are some methylene bridges on the structure of acrylic melamine that may have possibly been formed during the curing stage. Nguyen et al.17 showed that these methylene bridges break against water or humidity. The cleavage of the main linkage (etheric and methylene) acrylic melamine upon hydrolysis and the formation of various soluble species account for the occurrence of etching phenomenon (as seen in the microscopic images earlier).
Figure 4 reveals that the clearcoat that has only been exposed to 300 hrs weathering has higher-intensity and broader OH and NH peaks, as well as a lower etheric vibration peak, compared with the coatings that experienced both weathering and biological materials. These variations mean that the former has experienced more extensive hydrolysis reactions than the latter. Such a result is not in agreement with the microscopic images. The microscopic images showed greater etching of clearcoats exposed to both weathering and biological substances, implying a higher occurrence of hydrolytic reactions. This contradiction may be attributed to the greater etching of areas with lower crosslink density (having higher NH and OH and lower etheric bonds) and lesser etching areas with higher crosslink density (having lower NH and OH and higher etheric bonds). Therefore, in FTIR spectra obtained by removing a small sample from the clearcoat surface, the contribution of higher crosslinking density areas becomes stronger, showing lower NH and OH, and higher etheric bonds. More details have been discussed elsewhere.5
This figure shows that carbonyl groups of all coatings broaden and increase during weathering. For coatings exposed to weathering and biological together, this increase is more significant compared to samples that only experienced weathering. These suggest that biological substances do not hinder the effect of UV radiation. The plausible explanation by which the UV radiation influences the underneath coating is not clear and requires further study.
As stated before, the main contribution of such a profound degradation may be due to the effect of biological materials. The main objective of this study was to elucidate the source of such degradations caused by the biological materials used. Our observations show that the etching can be the result of a catalyzed hydrolytic degradation. Three hypotheses are probable: (1) an acid-catalyzed hydrolysis, (2) metal ion-catalyzed hydrolysis, and (3) an enzymatically induced hydrolysis. We have tried to explain the possibility of all these hypotheses as follows.
Acid-Catalyzed Hydrolysis
Acid-catalyzed hydrolysis has been investigated in several works.18-20 The degradations caused by acid rain, which are very common in urban and industrial areas, are included in this type of hydrolysis. It has been found that acid rain and acid-catalyzed hydrolysis are most likely to occur in moderate to strong acidic environments. For example, the results reported by Schulz et al.19 showed that the pH of a real acid rain in aggressive environments (Jacksonville, FL) existed in the range of 3.5-4.5. Also, they employed a method by which the harsher condition (even pH = 1.5) was applied to different clearcoats to evaluate their resistance to acid rain. The coatings were subjected to the synergistic effect of UV and acid rain, using acidic dew and fog (ADF) cycles, consisting of spraying a mixture of sulfuric, nitric and hydrochloric acid (1:0.3:0.17 equals to pH = 1.5).7,8
In order to explore the occurrence of this probable mechanism, the pH of pancreatin and bird droppings were measured. The measured pH values were 6.3 and 6.25, respectively. This shows that these materials are not acidic enough to engage the coating to hydrolyze. Apart from this pH difference, there is another reason for the absence of hydrolysis due to this hypothesis. It is related to the time required to cause such a phenomenon. By comparing the images obtained by Schulz et al., it can be demonstrated that the formation of an etched surface as severe as those presented in Figure 3 needs five 24-hr ADF cycles plus 2,000 hrs UV exposure, whereas the exposed time for pancreatin or bird dropping in our work was only 300 hrs. Also, our results showed that in pre-aged samples described elsewhere,11 such features can be produced even in shorter times (only 24 hr-exposure to pancreatin or bird dropping). Hence, this hypothesis seems weak to confirm the etching phenomenon.9
Catalysis by Metal Ions
Induced catalyzed hydrolysis due to metal ions occurs when some ionic species are available in the hydrolysis environment. The proposed mechanism for catalysis of metal ions is from one or a combination of the following approaches: (1) coordinating to the substrate and increasing the tendency toward a nucleophilic attack, (2) coordinating to the nucleophile (water) to enhance its reactivity to an electrophilic site, and (3) coordinating to “leaving groups” to facilitate its release from the intermediate.21-23 It has been shown that the most effective ions for this purpose are divalent 3D transition metals and trivalent lanthanide ions (Fe2+, Fe3+, Cu2+, Zn2+, Ni2+, Mn2+, Co2+, Pb2+, Eu3+, Lu3+, etc.) due to possessing high charge/size ratios. To check the presence of these elements and possibility of such a kind of hydrolysis, EDS analysis was carried out on the degraded and non-degraded areas of the clearcoat. These results are shown in Figure 5.
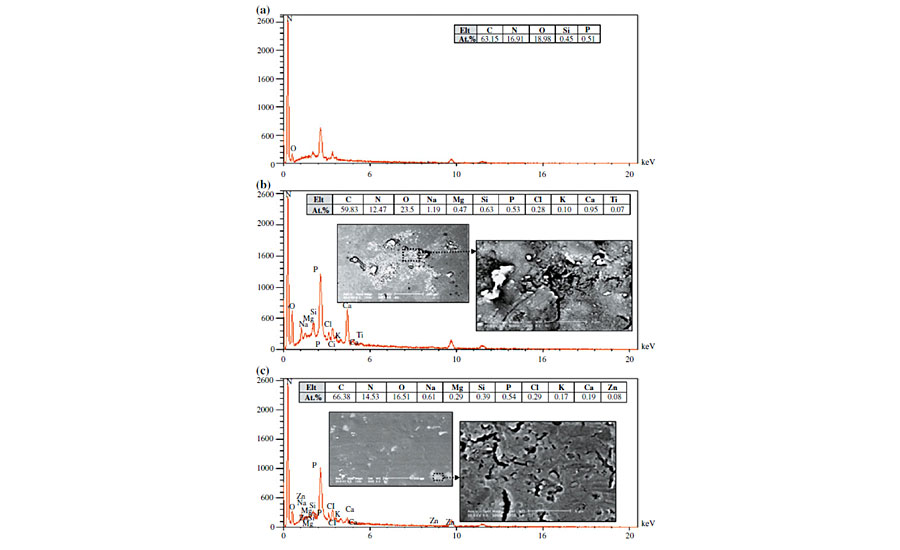
As seen in SEM images, it is observed that the clearcoat surface has not been degraded uniformly. Even a significant part of the clearcoat surface remained unaffected. It is also observed that there are also brighter points scattered on the clearcoat. Figure 5a shows EDS results of non-degraded or intact areas of a clearcoat exposed to biological materials. As expected for acrylic melamine, C, N and O are the main elements presented, and the existence of Si and P are attributed to silicon additives (leveling agent) and phosphoric acid derivatives (catalyst), respectively.
Figures 5b and 5c show EDS results of a bright point on clearcoats exposed to bird dropping and pancreatin, respectively. Elemental analysis of degraded areas caused by bird droppings and pancreatin showed that they are similar (except for Zn and Ti, which are relatively low). It was observed that the degraded areas exhibited no divalent 3D transition metals and trivalent lanthanide elements. As stated, these ions are able to catalyze hydrolysis reactions considerably. For the degraded areas influenced by both bird droppings and pancreatin, besides the main elements from acrylic melamine coating, the presence of Na, K, Ca and Mg elements is obvious. These are alkaline and alkali earth elements. It is well established in the literature that alkaline and alkali earth ions, due to their low charge/size ratio, do not show any catalytic effect.
In addition, XRF analysis of biological substances (shown later) indicated that bird droppings and pancreatin powders contain a high portion of Na, K, Ca and Mg, and also a relatively low amount of divalent 3D transition metals and trivalent lanthanide elements. There may be another source for metal ions from tap water used for preparation of biological slurries. (The absence of metallic elements in the intact areas of clearcoat (Figure 5a) and the fact that etching was not observed on clearcoats in weathering experiments, disproved the effect of metal ion catalysis caused by tap water.
Therefore, some complementary experiments were carried out to further examine the possibility of metal ion catalysis. Adding some foreign ions (e.g., NaCl solution, 0.1 M) to biological slurries showed no more effect compared to those without foreign ions.
These findings may show that although the metal ions have a weak contribution to catalyze the hydrolysis process, these apparently cannot be considered as the main degrading factor. More details on the effect of metal ions in degradation will be discussed further in this article.
Enzymatically Induced Hydrolysis
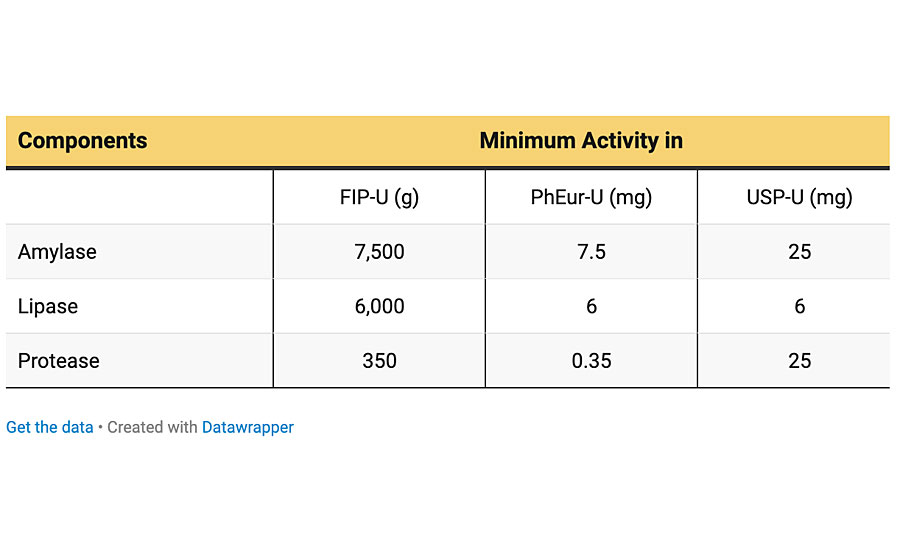
Enzyme-catalyzed hydrolysis is a common process in biological environments. Recently, such catalyzed reactions have been progressively used to biodegrade various synthetic polymers through the enzymes produced by microorganisms. Enzymes are amino acid molecules and their function is to catalyze various chemical reactions in biological environments, e.g., in the human body or animals. The rate of most enzyme-catalyzed reactions is millions of times faster than those of comparable uncatalyzed reactions. Hydrolase enzymes such as amylase, lipase, protease, etc. are the most important types of enzymes that catalyze hydrolytic reactions.
As stated before, due to the structural complexity and variability of bird droppings, the synthetic pancreatin was used to follow the degradation mechanism. There are different reasons to accept the similarity between bird droppings and pancreatin. However, a slight difference can be imagined.10,11
FTIR spectra of both biological substances reveal that although there are slight differences, the chemical structures are generally similar (Figure 6). Also, the elements presented in pancreatin and bird droppings are reasonably the same. Beside these analytical studies, the identical features of damaged clearcoats resulted from exposure to bird droppings and pancreatin (Figure 3), may show their similar influence.
It has been reported that pancreatin consists of amylase, lipase and protease, which are all hydrolase enzymes and are responsible for cleavage of C–O–C (e.g., in starches), COO esteric linkage (e.g., in glycerin) and CO–NH peptide amide linkages (e.g., in proteins), respectively. Since the bird droppings have a complex structure and contain impurities, it is hard to find their ingredients. However, based on similar results obtained here, it is plausible to accept that these two biologicals can be used interchangeably. In other words, the mechanism of degradation for pancreatin may be the same as bird droppings. In addition, since the main part of bird nutrition is carbohydrates, such as cereals and starch-based foods, the presence of the above-mentioned enzymes would be inevitable. The specifications of pancreatin used is presented in Table 1.
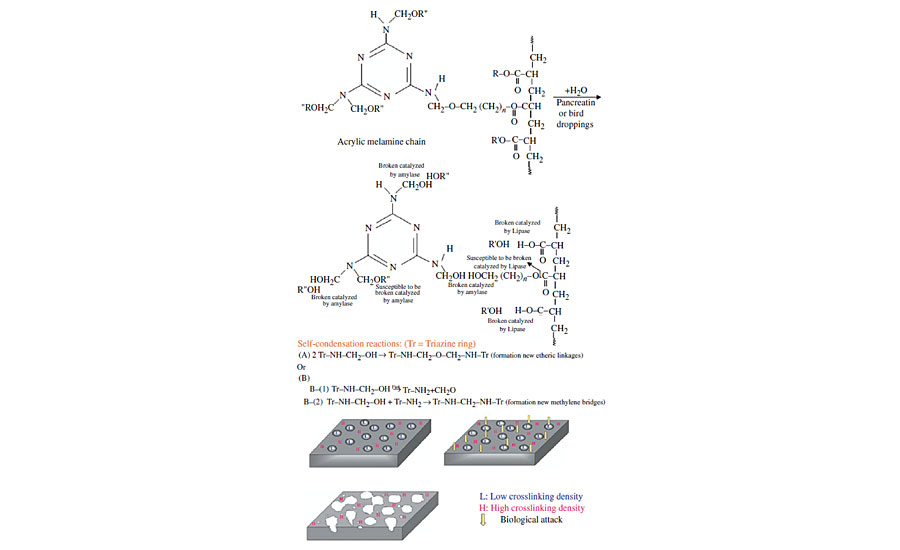
Based on these results, the following mechanism can be attributed to the degradation of acrylic melamine in contact with bird droppings or pancreatin (Figure 7). After pancreatin or bird droppings are deposited on a clearcoat surface, the hydrolysis reaction can take place. The enzymes present in these materials catalyze the hydrolysis reaction. Among these enzymes, protease due to the absence of amide linkages (–CONH–) is relatively inactive on acrylic melamine. However, amylase and lipase enzymes act on etheric and esteric linkages respectively, accelerating the cleavage of these bonds. Due to the presence of high active sites (etheric and esteric linkages) in acrylic melamine, the crosslinked network is cleaved at their active sites, leading to the formation of soluble products and releasing from the coating, leaving an etched area on the surface. The clearcoat consists of high-crosslinking and low-crosslinking areas. The latter are more vulnerable against hydrolytic degradations,17,32 and are more affected than the former. The existing dangling groups on the clearcoat can either remain unreacted or can self-condense with each other (as depicted in Figure),12 forming new etheric or methylene linkages. The existence of these new etheric linkages in FTIR spectra of degraded samples is not easily detectable (because newly formed etheric linkages are less than those broken before).
In order to investigate the influence of temperature on degradation and to determine the temperature at which enzyme becomes denatured, the clearcoats were exposed to pancreatin at various temperatures (20, 40, 60 and 80 °C) for 3 hrs, and their damaged surfaces were evaluated by optical and SEMs. These images are exhibited in Figure 8.

Both macroscopic and microscopic images show a same trend. It is observed that by increasing the temperature up to 60 °C, the intensity of degradation increases, but after that the clearcoat surface experiences less degradation. The effect of temperature may confirm the previous conclusion, in which the acid and the metal ion catalysis mechanisms were not involved for such degradation. The reason is that by increasing the temperature without any limitation, the acidic and metallic catalytic effects increase.16 But it seems inapplicable here, as the temperature has a negative effect on catalytic role of biological materials. The decreased degradation at 80 °C may be due to partial denaturing of enzymes presented in pancreatin. The results obtained in an earlier study have shown that in the presence of calcium ion, the denaturation temperature of amylase increases from 48° to 83 °C. It has also been reported that the optimum activation temperature of lipase varies between 56° and 100 °C, depending on the presence of metal ions and the aqueous or non-aqueous environment utilized. Since the presence of calcium was observed in pure pancreatin (as evidenced by XRF analysis in Figure 6) as well as in the damaged areas (Figure 5), this high denaturation temperature is plausible. These results indicate that the onset point of denaturation seems to be somewhere between 60° and 80 °C, implying that pancreatin is still active at 60 °C (the used temperature in this study) and can still continue its role at this temperature.13
It is known that some metal ions, e.g., Na+, K+, Ca2+ and Mg2+ activate the enzyme. Since in the degraded area these were present (Figure 5), we tried to probe the origin of these elements. To check whether they come from the water added in preparation of biological slurries or due to the presence of biological materials, deionized (DI) water was also used to prepare the slurries. Degradation of clearcoat by pancreatin slurries prepared by DI water showed that the origin of these metal ions is from pancreatin not from the tap water. This result was further confirmed by XRF analysis of pancreatin powder, which showed the existence of those elements. In order to examine whether these elements are coordinated to enzymes or are free ions in aqueous solution, pancreatin powder was washed by DI water, followed by centrifuging at high speed several times. The rinsed pancreatin was dried and the EDS analysis was again carried out. The EDS results of pancreatin before and after repeated rinsing with DI water are given in Table 2.

Table 2 indicates that some of the elements that were detected by XRF are undetectable by EDS due to their very low content. It is observed that elements such as Na, Mg and Ca are present even after rinsing with DI water, implying that these elements may have been coordinated to pancreatin (or enzymes). The slurry of the rinsed pancreatin and DI water was deposited on clearcoat surface at 60 °C for 24 hrs. After removing the pancreatin, the clearcoat surface was characterized by SEM and EDS analysis. These results are presented in Figure 9.
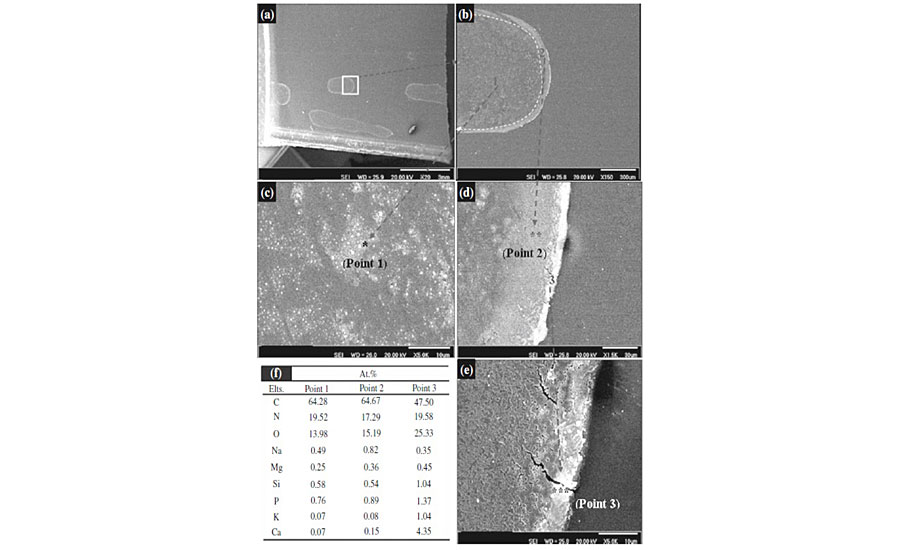
Figure 9a shows a part of degraded area that exhibits a large, stain-like region. These show the macroscopic-degraded areas. There also seems to be a trace of large slurry droplets. By focusing on these droplets (Figure 9b), it can be clearly demonstrated that there are at least three distinguishable parts within the stains (point 1), near to edge (point 2) and exactly at the edge of the stain (point 3). The highly magnified images of these points are depicted in Figures 9c, 9d and 9e. SEM micrographs demonstrated that as we move far from the center toward the edge of the stain, the intensity of degradation and crack formation becomes greater.
EDS analysis of these points (Figure 9f) reveals that the concentration of metals increases from center toward the edges. Since it was shown that metal species are coordinated to enzyme molecules, the greater presence of metal species may be an indication of a higher existence of enzymes. The greater concentration of enzyme in edges can be attributed to the tendency of hydrophobic part of enzymes, causing a driving force toward edges of the droplet. This also may be assigned to a process called “coffee stain effect,” in which due to greater evaporation at the edges, a flow drives the enzymes from the center toward edge of droplets.36 The greater concentration of enzyme, the higher the chance of sticking to substrate and the greater degradation at the edge of the stain. Figure 9c shows that the clearcoat surface has not been degraded uniformly. There are distributed micro-scale degraded and intact areas. These uneven degraded and intact areas may be indicative of micro-aggregation of enzyme molecules that stick to the clearcoat surface and catalyze hydrolytic degradation of that point.
As seen in Fig. 9e, there are some cracks at the edges. This may be attributed to the metal ion-induced oxidation due to the presence of metal ions and the abundance of oxygen at the edges of droplet.
In this manner, the enzymatically induced hydrolysis is believed to be the main mechanism involved in degrading acrylic melamine clearcoats caused by bird droppings.
Conclusion
Our study attempted to reveal the degradation mechanism of a typical acrylic melamine automotive clearcoat caused by bird droppings. Natural bird droppings along with an equivalent synthetic material (pancreatin) were used. XRF and FTIR proved the similarity of these two. FTIR analysis of clearcoat showed that the catalytic hydrolysis of etheric and esteric bonds was the reason for such degradation. Three different mechanisms were proposed: acid-catalyzed, metal ion-catalyzed and enzymatic-catalyzed hydrolysis. It was concluded that due to a relatively neutral environment of bird droppings and pancreatin, and the absence of metal ions at the degraded areas, the first and second hypotheses have little or no contribution in such degradations. It was found that natural bird droppings and pancreatin, due to containing some digestive hydrolyze enzymes such as amylase and lipase, are able to catalyze the hydrolytic cleavage of etheric and esteric linkages of acrylic melamine clearcoats. The consequence of these cleavages is the release of water-soluble products from the coating, leaving etched areas and local defects as well as a decreased appearance on the clearcoat surface.
References
¹ Stevani, C.V;, Porto, J.S.; Trindade, D.J.; Bechara, J.H. Mechanism of Automotive Clearcoat Damage by Dragonfly Eggs Investigated by Surface Enhanced Raman Scattering. Polym. Degrad. Stab. 61-66 (2000).
2 PSA PEUGEOT-CITROE¨ N test method D27 5415, Paint Coatings Resistance to Biological Attacks.
3 Ramezanzadeh, B.; Mohseni, M.; Yari, H.; Sabbaghian, S. A Study of Thermal-Mechanical Properties of an Automotive Coating Exposed to Natural and Simulated Bird Droppings. J. Therm. Anal. Calorim. s(in press). doi:10.1007/s10973-009-0442-4.
4 Ramezanzadeh, B.; Mohseni, M.; Yari, H.; Sabbaghian, S. An Evaluation of an Automotive Clear Coat Performance Exposed to Bird Droppings Under Different Testing Approaches. Prog. Org. Coat.,149-160 (2009).
5 Yari, H.; Mohseni, M.; Ramazanzade, B.; Naderi, N. Use of Analytical Techniques to Reveal the Influence of Chemical Structure of Clearcoat on Its Biological Degradation Caused by Bird-Droppings. Prog. Org. Coat., 281-290 (2009).
6 Bauer, D.R. Degradation of Organic Coatings I. Hydrolysis of Melamine Formaldehyde/Acrylic Copolymer Films. J. Appl. Polym. Sci., 3651-3662 (1982).
7 Mori, K.; Tachi, K.; Muramatsu, M.; Torita, K. Mechanism of Acid Rain Etching of Acrylic/Melamine Coatings. Prog. Org. Coat., 34-38 (1999).
8 Schulz, U.; Trubiroha, P.; Schernau, U.; Baumgart, H. The Effects of Acid Rain on the Appearance of Automotive Paint Systems Studied Outdoors and in a New ArtificialWeathering Test. Prog. Org. Coat., 151-165 (2000).
9 Palm, M.; Carlsson, B. New Accelerated Weathering Tests Including Acid Rains. J. Coat. Technol., 69-74 (2002).
10 Calil, M.R.; Gaboardi, F.; Bardi, M.A.G.; Rezende, M.L.; Rosa, D.S. Enzymatic Degradation of Poly(e-Caprolactone) and Cellulose Acetate Blends by Lipase and a-Amylase. Polym. Test., 257-261 (2007).
11 Kurokawa, K.; Yamashita, K.; Doi, Y.; Abe, H. Surface Properties and Enzymatic Degradation of End-Capped Poly(L-Lactide). Polym. Degrad. Stab., 1300-1310 (2006).
12 Sangaj, S.; Malshe, V.C. Permeability of Polymers in Protective Organic Coatings. Prog. Org. Coat., 28-39 (2004).
13 Polaina, J.; MacCabe, A.P. Industrial Enzymes: Structure Function and Applications. Springer, Netheralnds (2007).
14 Schandl, A.; Pittner, F. The Role of Na+ and Ca2+ Ions on the Action of Pancreatic Lipase Studied with the Help of Immobilisation Techniques. Eur. J. Biochem., 547-551 (1984).
15 Nguyen, T.; Martin, J.W.; Byrd, E; Embree, N. Relating Laboratory and Outdoor Exposure of Coatings III. Effect of Relative Humidity on Moisture-Enhanced Photolysis of Acrylic-Melamine Coatings. Polym. Degrad. Stab., 77 1-16 (2002).
16 Yari, H.; Moradian, S.; Ramezanzadeh, B.; Kashani, A.; Tahmasebi, N. The Effect of Basecoat Pigmentation on Mechanical Properties of an Automotive Basecoat/Clearcoat System During Weathering. Polym. Degrad. Stab., 94 1281-1289 (2009).
17 Martin, J.W.; Nguyen, T.; Byrd, E.; Dickens, B.; Embree, N. Relating Laboratory and Outdoor Exposures of Acrylic Melamine Coatings I. Cumulative Damage Model and Laboratory Exposure Apparatus. Polym. Degrad. Stab., 75 193-210 (2002).
18 Yari, H.; Mohseni, M.; Ramezanzadeh, B. Comparisons of Weathering Performance of Two Automotive Refinish Coatings: A Case Study. J. Appl. Polym. Sci., 111 2946-2956 (2009).
19 Tahmassebi, N.; Moradian, S. Predicting the Performances Of Basecoat/Clear Coat Automotive Paint Systems by the Use of Adhesion, Scratch and Mar Resistance Measurements. Polym. Degrad. Stab., 83 405-410 (2004).
20 Gerlock, J.L.; Smith, C.A.; Cooper, V.A.; Dusbiber, T.G.; Weber, W.H. On the Use of Fourier Transform Infrared Spectroscopy and Ultraviolet Spectroscopy to Assess the Weathering Performance of Isolated Clearcoats From Different Chemical Families. Polym. Degrad. Stab., 62 225-234 (1998).
21 Gaszner, K.; Neher-Schmitz, H.; Kuntz, T. A Report by Forschungsinstitut for Pigment and Lake eV (FPL), pp. 1-4. Global Automotive Manufacturing & Technology (2003).
22 Ramazanzade, B.; Moradian, S.; Yari, H.; Kashani, A.; Niknahad, M.; Chadle, H.; Motamed, M.; Adeli, M.; Shirakbari, N. The Effect of Basecoat Pigmentation on Weathering Performance of an Acrylic/Melaminebasecoat/Clearcoat Automotive Finishes. Proceeding of Automotive Adhesive, Sealants and Coatings, Stuttgart, Germany, 2008.
23 Nguyen, T.; Martin, J.W.; Byrd, E. Relating Laboratory and Outdoor Exposures of Acrylic Melamine Coatings IV. Mode and Mechanism for Hydrolytic Degradation of Acrylic- Melamine Coatings Exposed to Water Vapor in the Absence of Light. J. Coat. Technol., 75(941) 37-50 (2003).
24 Nguyen, T.; Martin, J.; Byrd, E.; Embree, N. Relating Laboratory and Outdoor Exposure of Coatings II. Effects of Relative Humidity on Photodegradation and the Apparent Quantum Yield of Acrylic Melamine Coatings. J. Coat. Technol., 74(932) 65-80 (2002).
25 Huang, C.; Stone, A.T. Hydrolysis of Naptalam and Structurally Related Amides: Inhibition by Dissolved Metal Ions and Metal (Hydr)Oxide Surfaces. J. Agric. Food Chem., 47 4425-4434 (1999).
26 Huang, C.; Stone, A. Synergistic Catalysis of Dimetilan Hydrolysis by Metal Ions and Organic Ligands. Environ. Sci. Technol., 34 4117-4122 (2000).
27 Smolen, J.M.; Stone, A.T. Divalent Metal Ion-Catalyzed Hydrolysis of Phosphorothionate Ester Pesticides and Their Corresponding Oxonates. Environ. Sci. Technol., 31 1664-1673 (1997).
28 Zago´rowska, I.; Kuusela, S.; Lo¨nnberg, H. Metal Ion- Dependent Hydrolysis of RNA Phosphodiester Bonds Within Hairpin Loops. A Comparative Kinetic Study on Chimeric Ribo/24-O-Methylribo Oligonucleotides. Nucl. Acid Res., 26 3392-3396 (1998).
29 Brueckner, T.; Eberl, A.; Heumann, S.; Rabe, M.; Guebitz, G.M. Enzymatic and Chemical Hydrolysis of Poly(Ethylene Terephthalate) Fabrics. J. Polym. Sci. A: Polym. Chem., 46, 6435-6443 (2008).
30 Nathalie, V.; Lalot, T.; Brigodiot, M.; Mare´chal, E. Enzyme- Catalyzed Hydrolysis of Unsaturated Polyester Networks. I. Study of the Hydrolysis of a Precursor: Poly(1,2-Propanediyl Fumarate). J. Polym. Sci. A: Polym. Chem., 35 27-34 (1997).
31 Nathalie, V.; Lalot, T.; Brigodiot, M.; Mare´chal, E. Enzyme- Catalyzed Hydrolysis of Unsaturated Polyester Networks. II. Enzyme-Catalyzed Hydrolysis of Polyester Networks Prepared From Poly(1,2-Propanediyl Fumarate). J. Polym. Sci. A: Polym. Chem., 35 35-40 (1997).
32 Xi, Z.; Yoshida, T.; Funaoka, M. Enzymatic Degradation of Highly Phenolic Lignin-Based Polymers (Lignophenols). Eur. Polym. J., 39 909-914 (2003).
33 Montaudo, G.; Rizzarelli, P. Synthesis and Enzymatic Degradation of Aliphatic Copolyesters. Polym. Degrad. Stab., 70 305-314 (2000).
34 Nielsen, A.D.; Fuglsang, C.C.; Westh, P. Effect of Calcium Ions on the Irreversible Denaturation of a Recombinant Bacillus halmapalus a-Amylase: A Calorimetric Investigation. Biochem. J., 373 337-343 (2003).
35 Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature, 389 827-829 (1997).
36 Ratner, B.; Haffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science. An Introduction to Materials in Medicine, 1st ed. Academic press, New York (1997).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









