Spectroscopic Analysis of Architectural Coatings for Improved Weathering

New product development or major reformulation of high-durability exterior coatings is among the highest-risk projects a coatings lab can undertake. This is due to the years-long exterior exposure time required to validate a new product’s performance, in addition to the standard formulation and test cycle time of several months. There are many attributes associated with the assessment of a coating’s weatherability, such as grain crack resistance, adhesion, chalking, etc. In the architectural semi-gloss to high-gloss space, an emphasis has rightly been placed on gloss retention as a distinguishing attribute between high-performance and economy product classes.
Loss of gloss from exposure leads to not only a change in the desired aesthetic, but also provides the first visual indication of polymer degradation, and therefore, the initiation of additional property loss. It is important that the formulator utilizes all tools available to build confidence in their coatings as early in the formulation screening stage as possible, to ensure the right samples are selected for long-term, real-world exposure studies. We have employed a spectroscopic technique that differentiates UV durability on the order of weeks rather than months or years. This method uses time-resolved functional group analysis by ATR-FTIR and acts as an indicator of relative gloss durability in acrylic coatings.
Current Gloss Retention Methods
The gold standard for determining gloss retention is to expose a series of coatings to actual exterior conditions for a minimal period of 6-24 months. This is an intuitive test that involves painting a relevant substrate, putting it outside (preferably in a harsh environment) and measuring the gloss over time. While simple, it is a very strong test due to its real-world applicability and ability to test many different properties at once, such as dirt pickup resistance, grain crack and mildew resistance. An ideal exposure would incorporate multiple formula variations, colors, panel orientations, geographic locations and replicates. This type of approach becomes prohibitively large, namely when multiple bases are tested simultaneously and for multiple years. For this reason, accelerated methods are used to refine formulations and reduce the complexity of an exposure series.
The most widely used accelerated method is cyclic UVA, where samples are placed in weathering cabinets and exposed to alternating humidity and UV conditions, such as the ASTM G154 cycle. UVA light emits a wavelength of 340 nm, which matches the primary wavelength responsible for organic degradation by solar-emitted radiation. An alternative to cyclic UVA is cyclic UVB, where the primary irradiation wavelength is 313 nm instead of 340 nm. This higher-energy wavelength is harsher than UVA but does not match sunlight’s UV profile particularly well. As a result, UVB is not conventionally used for acrylic coatings and is typically reserved for ultra-high-durability chemistries. Xenon Arc is a third approach to accelerated weathering. This UV profile closely matches the full spectrum of sunlight but is a newer approach that is not as widely used as cyclic UVA. In all cases, accelerated weathering methods require less time to evaluate compared to exterior exposure, yet can still take five months or longer to differentiate high-performance acrylic systems. Differentiating coatings earlier in the cyclic UVA method has the potential to greatly accelerate development cycle time.
Gloss Retention and Photodegradation
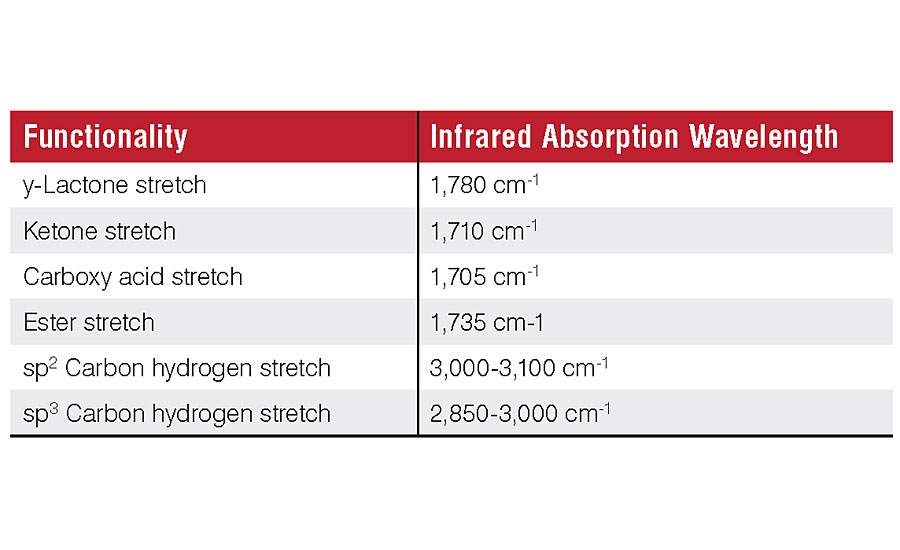
In order to create a quicker predictive method, it is important to understand the processes that occur in a coating when put outside. Many environmental factors influence how the gloss of a coating changes over time, including moisture, dirt pickup and temperature. The largest overall impact is generally caused by UV, especially in southern climates where UV indexes are highest, such as Florida, Texas and Southern California. When UV light irradiates a film it will abstract protons from the polymer, creating radicals. These radicals are highly reactive and readily form peroxy radicals, which result in polymer bonds breaking and various functional group transformations of carbonyl groups – including y-lactones, ketones, carboxy acids or esters. If this scission occurs on the backbone of the polymer, it creates two lower-molecular-weight products that more easily erode from the film. These molecular changes can be seen far earlier than the surface roughness changes detectable by gloss measurement. FTIR analysis can observe functional group changes in days or weeks rather than the months necessary for gloss analysis.
ATR-FTIR Characterization
Fourier Transform Infrared Spectroscopy (FTIR) is a spectroscopic technique used to determine the infrared absorption spectrum of a sample with the advantage of being able to cover a wide range of wavelengths quickly. Over the infrared region, the absorbances in a sample are caused by the infrared light at a specific wavelength, initiating movement in specific chemical bonds within the sample. In order to perform FTIR on a dry film sample, the Attenuated Total Reflectance (ATR) sampling technique is utilized. This technique uses the inherent total internal reflection of a sample, allowing for the characterization of the surface of solid samples.
The consequence of photo-oxidation of polymers in a paint film is the conversion of carbon-hydrogen bonds to carbon-oxygen (carbonyl) functionalities. ATR-FTIR is well suited to quantify these functional group changes. ATR-FTIR is a rapid, non-destructive test, and a single measurement can be completed in minutes. The technique has a small penetration depth of 0.5 to 2 micrometers. This focuses the data acquisition on the coating’s surface, where changes in surface roughness affecting gloss occur. The carbonyl stretch at 1,600-1,800 cm-1 and the sp2 and sp3 hybridized carbon-hydrogen stretches at 2,800-3,050 cm-1 can easily be resolved by this technique (Figure 1).
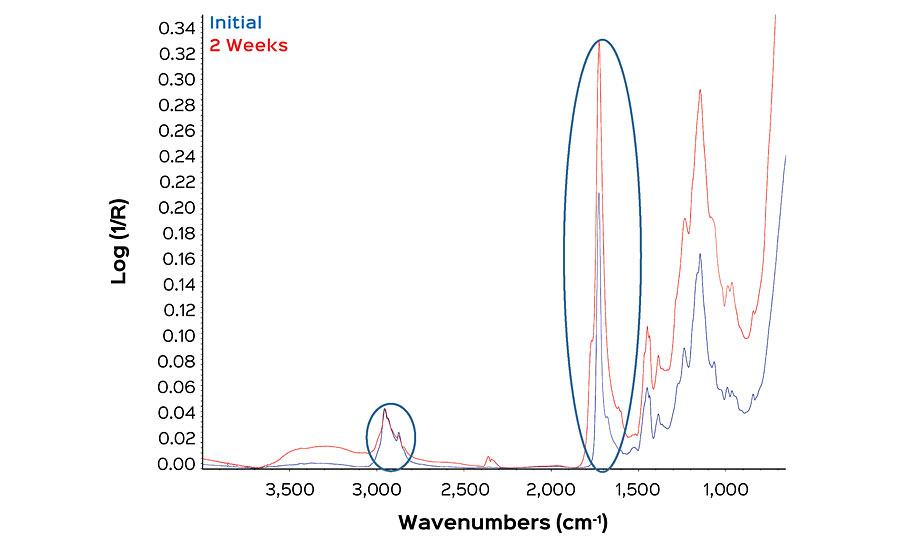
The oxidation state of a given film will be referred to as the “carbonyl index”. This value is defined as the % absorbance due to carbonyl stretching, divided by the % absorbance of the carbon-hydrogen stretch. This unitless value allows films to be assessed at different exposure times where a given exposure time can be subtracted from the initial carbonyl index to give the “delta carbonyl index” or DCI (Equation below).
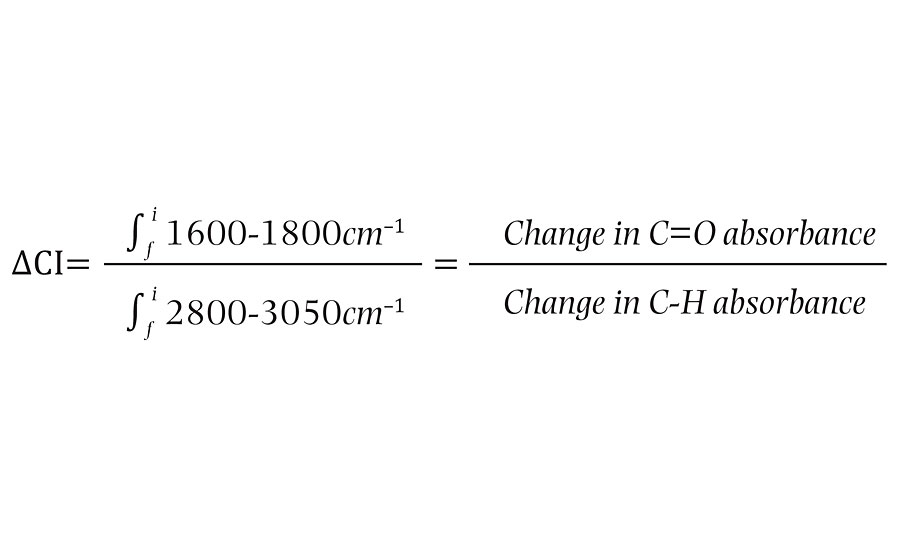
The delta carbonyl index shows how the ratio of carbonyl content has changed in that period and consequently, how much photo-oxidation has taken place between measurements (Table 1).
Method
All carbonyl indexes were measured using a Thermo Scientific Nicolet iS10 FTIR with a Smart iTR attachment and a ZnSe crystal. Samples were drawn down at 10 mil wet film thickness on 3 in. x 6 in. aluminum panels and cured overnight. The baseline carbonyl index was measured after overnight cure and then the panel was put into a Q-Lab QUV equipped with UVA lightbulbs. The carbonyl index was taken as the average of three FTIR scans taken at different locations within a panel. The integrated values of all spectra were averaged, and the carbonyl index was calculated. The QUV cycled between 8 hrs of UV exposure, with an irradiance of 0.89W/m2 and a temperature of 60 ºC, then 4 hrs of condensation at 50 ºC. Samples were removed from the QUV for individual measurements, then returned to the QUV as required.
Correlating Carbonyl Index and Cyclic UVA
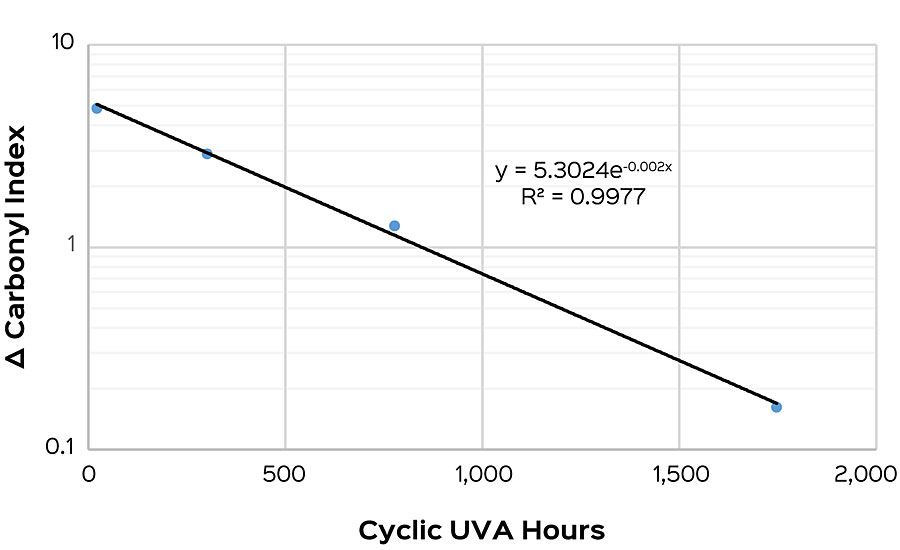
Tracking the carbonyl index of a sample allows an understanding of the photodegradation that is taking place, but it doesn’t necessarily provide the whole answer to gloss changes occurring in a film. Gloss changes can be caused by a variety of other factors such as mechanical, adhesive or dirt pickup failure modes. Carbonyl index is sensitive to only those factors that result in a change in FTIR absorbances. In many cases, however, photo-oxidation is the primary mechanism of film degradation that FTIR is well suited to detect. High-performance architectural coatings are designed to maintain their gloss, but after a sufficient amount of UV exposure, gloss typically will drop quickly. To establish correlation between gloss units as exposed to UV and carbonyl index, we assign an “onset of failure” point for each system, as seen in Figure 2. This failure point is defined as the longest exposure time prior to a substantial change in slope. In cases where a failure point is ambiguous, it is usually the case that photo-oxidation is not the only mechanism of failure and other modes need to be considered. The onset of failure is observed to correlate with the rate of photodegradation within a sample, where lower indexes indicate slower rates of degradation.

To study this, four modifications were made to a base polymer to intentionally induce a range of photodegradation rates. These polymers were then formulated into a high-gloss pastel base. Samples were put into cyclic UVA and had their gloss and carbonyl index measured weekly. After 2,750 hrs, the onset of failure was determined for each sample. This was compared to the carbonyl index delta taken after 7 days for each polymer, and then fit with an exponential regression. An R2 of 0.9977 was found, suggesting a good correlation between the onset of failure and carbonyl index (Figure 3).
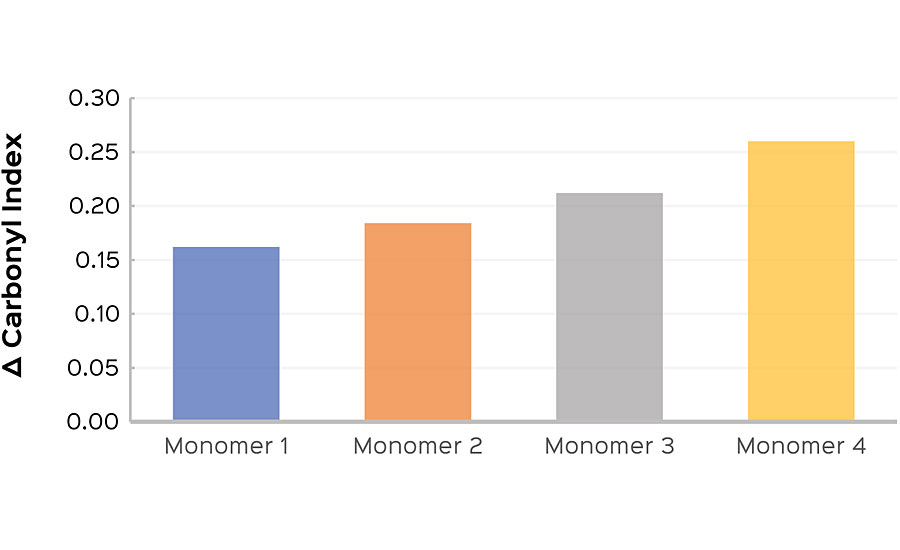
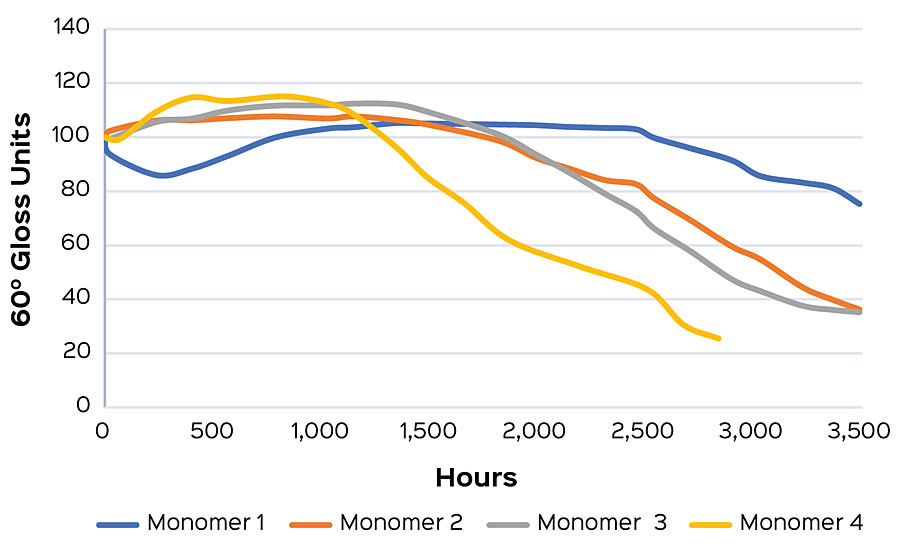
This work is currently utilized in the EPS lab in order to create exterior polymers with market-leading gloss retention. In a recent monomer comparison experiment, a primary monomer was swapped for three alternatives, adjusting to maintain constant Tg. The resulting coatings were exposed to UVA in the method described above, and the change in carbonyl index delta was determined at 24 hrs for each sample. A higher carbonyl index delta indicates a higher degree of film oxidation. A sample showing higher relative values would be expected to show early gloss loss compared to a sample with a lower carbonyl index delta. In this study, Monomer 1 had the lowest delta and was predicted have the best long-term gloss performance after 24 hrs of cyclic UV exposure, followed by Monomers 2 and 3, respectively. Monomer 4 showed the highest carbonyl index delta, and was predicted to have earlier gloss loss than the other samples in the series (Figure 4a). Gloss was measured weekly for an additional 21 weeks of cyclic testing. After that time it was found that the gloss performance matched the prediction made from the 24 hr carbonyl indexes (Figure 4b).
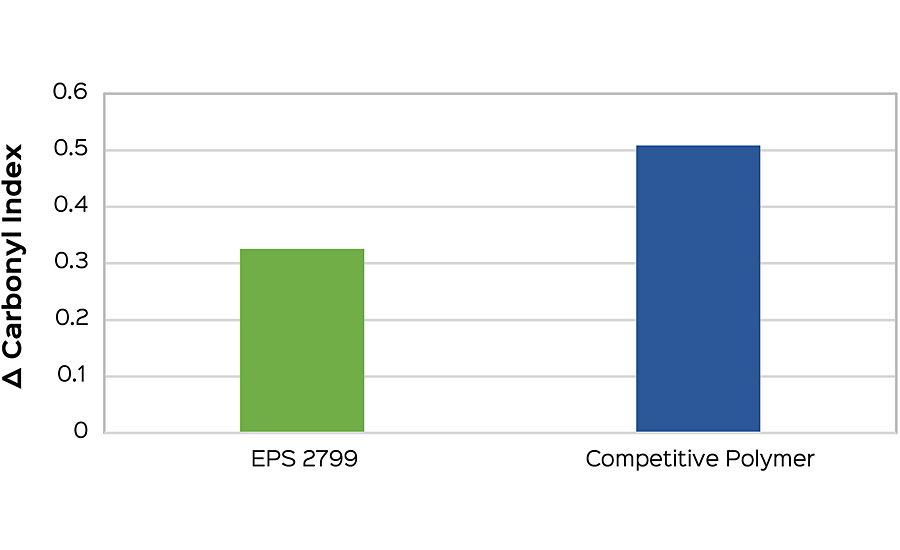
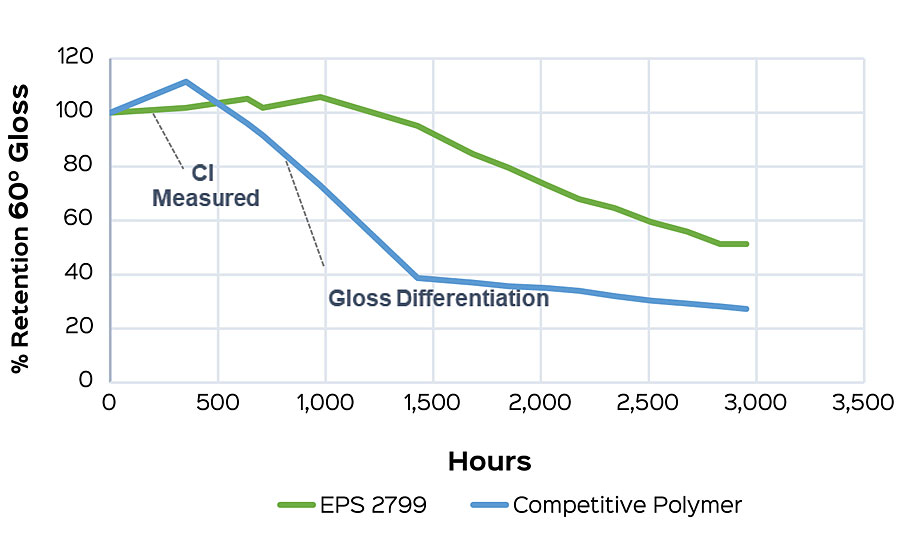
This method also allows for early comparisons to be made when screening polymers for a formulation. Formulation can have a strong influence on the carbonyl index, and so the best interpretations will come when compositional differences are minimized. As an example, a high-gloss white formulation was tested to compare EPS® 2799 and another high-performance, high-gloss polymer. Carbonyl indexes were taken at one week (168 hrs), and the difference from the initial was calculated. The low carbonyl index delta of EPS 2799 (Figure 5a) indicates a reduced susceptibility to photo-oxidation compared to the commercial alternative. This performance conclusion is confirmed after an additional five weeks of testing after onset of failure is seen in both samples (Figure 5b).
Accelerating Development
Real-world exposure testing will always be required to validate exterior performance. The coatings industry has relied on accelerated methods like cyclic UVA as an excellent way to reduce the formula candidate pool for long-term testing, though it still requires months of exposure time. We have shown that by incorporating carbonyl indexing using ATR-FTIR, cyclic UVA testing can provide valuable formula differentiation on gloss retention far earlier than direct gloss measurement. ATR-FTIR offers an attractive incentive to gain more from UVA testing and has the potential to accelerate product development on exterior products.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






