UV-Vis-Resistant Acrylic Latexes for Wood Coating Applications
by Encapsulation of Inorganic NPs Using Mini-Emulsion Polymerization

Clear coatings containing UV absorbers (UVAs), hindered amine light stabilizers (HALS) and biocides can substantially slow down the weathering process of wood and maintain its natural color for a period of time.1 However, there are few commercially available coatings that can push refinishing intervals past two or three years. Currently, wood in exterior applications with translucent coatings requires frequent refinishing and maintenance,2 which limits competitiveness with alternative materials. One cause of the frequent maintenance is the premature photodegradation of additives in coatings. The additives currently used in clear coatings to protect wood against ultraviolet (UV) radiation and black-stain are mostly organic and are often vulnerable to photodegradation, which, along with volatilization and leaching, reduce their long-term efficacy. As additive efficiency decreases, coatings and wood will break down, creating micro-cracks or small perforations that facilitate fungal colonization.3 All of this results in the loss of wood’s aesthetic properties.
One approach to improve the coating and wood photostabilization is to replace organic UVAs with inorganic metal oxide nanoparticles such as ZnO, TiO2 and CeO2.3,4 Several studies have investigated this approach in wood coating applications. Weichelt et al. (2010)5 and Auclair et al. (2011)6 report the effectiveness of ZnO nanoparticles in slowing down wood discoloration and delamination of UV-cured coatings. Similar results are presented by Cristea et al. (2011)7 where the addition of ZnO and TiO2 nanoparticles in an acrylic stain slows down photodegradation. However, these studies also reported that nanoparticles tend to aggregate and sediment, leading to adhesion problems. Since the performance of nanocomposites can be greatly influenced by the dispersion, there is a need to improve nanoparticle dispersion in coatings.
Mini-emulsion polymerization can be used to obtain better dispersion of inorganic material in the polymeric matrix and also to reduce the nanoparticle aggregation in the film.8 Mini-emulsion polymerization is a powerful technique to obtain hybrid organic-inorganic materials in which nanoparticles are incorporated into or onto the polymer particles.9 In our previous work, it was shown that silver nanoparticles could efficiently be encapsulated in the polymer matrix in order to develop a coating resistant to black-stain fungi.10 This study aims to use mini-emulsion polymerization to encapsulate inorganic UVAs in order to improve photostabilization of exterior wood coatings. Zinc oxide nanoparticles were selected in three different sizes, in order to address the performance in function of nanoparticles size. The performances of the synthesized latex containing inorganic UVAs were compared with a control formulation containing a commercial UV absorber. The comparison between the various systems was based on color and gloss retention, Fourier-transform infrared (FTIR) spectroscopy and variation in the glass transition (Tg).
Experimental Methods
Methyl methacrylate (MMA), butyl acrylate (BuA) and acrylic acid (AA) were obtained from Sigma-Aldrich and were purified on a basic aluminum oxide column before use to remove the inhibitor. Oleic acid, hexadecane (HD), sodium dodecylsulfate (SDS, 99%) and potassium persulfate (KPS, 99%), also from Sigma-Aldrich, were used as received. Tinuvin 1130 (benzotriazole family compounds), Tinuvin 292 (mix of 1,2,2,6,6-pentamethyl- 4-piperidyl sebacate compounds) and the acrylic binder (Acronal 4110) were obtained from BASF. Zinc chloride was obtained from Alfa easer and AMP-95 from ANGUS Chemical.
Inorganic Nanoparticle Synthesis and Functionalization
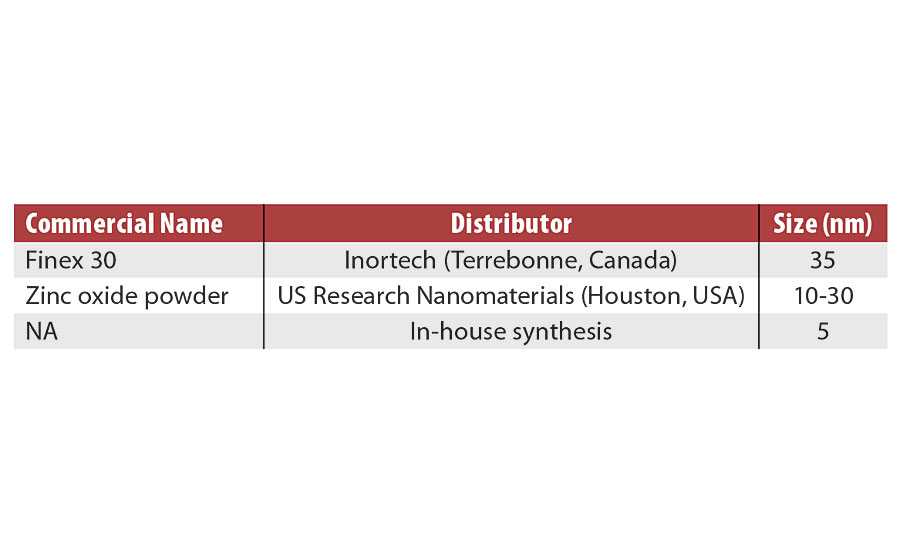
For this work, zinc oxide nanoparticles with three different sizes were selected; two are available commercially and one was synthesized. Their characteristics are presented in Table 1. The commercial nanoparticles were received in powder form. In order to be easily dispersed in the organic phase, the ZnO powders were functionalized with oleic acid. Moreover, 5 nm functionalized zinc oxide nanoparticles were synthesized based on the work of Mikulski et al. (2016).11 First, 10 mmol of zinc chloride and 3 mmol of oleic acid were added to 100 mL of ethanol. Then, a NaOH solution (30 mmol in 100 mL of ethanol) was added to the zinc chloride solution at room temperature with vigorous stirring. The mixture was heated to 45 °C and held for 8 hrs. The synthesized oleic acid-coated ZnO nanoparticles were centrifuged (3,000 rpm, 15 min, 20 °C) and then purified three times with ethanol; subsequently, the nanoparticles were dispersed in hexanes.
Latex Synthesis and Formulation Preparation
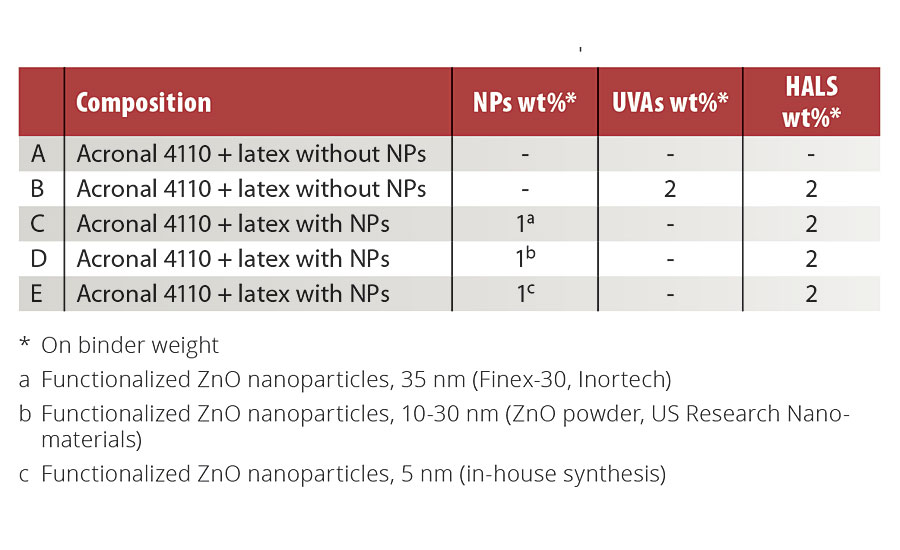
The previous ZnO nanoparticles were incorporated into the latex polymer particles using mini-emulsion polymerization. Firstly, monomers and 1% (on monomers weight) of hydrophobic agent (hexadecane) were mixed together in a beaker. In a second beaker, 30 mL of surfactant (SDS) was dissolved in Nanopure™ water. The organic phase containing a predetermined amount of nanoparticles was added to the aqueous phase, and the resulting solution was stirred mechanically for 1 hr. The mixture was sonicated using an ultrasonic probe for 3 min at an amplitude of 80%. Simultaneously, 2% (on monomers weight) of initiator (KPS) was dissolved in 8 mL of Nanopure water. Afterwards, the mini-emulsion and the KPS solution were loaded in a three-neck round-bottom flask and purged under nitrogen for 20 min. The system was heated under reflux for 3 hrs at 70 °C. The quantities of each component were calculated in order to obtain a total volume of 40 mL and 15% solid content. Formulations containing 1% (on binder weight) of ZnO nanoparticles were prepared. As the solid content of the synthesized latexes was quite low compared to commercially available resins, the synthesized latexes were mixed in a 1:1 mass ratio with an acrylic commercial resin (Acronal 4110 from BASF) with solids content between 49-51%. The final solids content of the prepared formulations was 33%. As a reference, a formulation (B) was prepared with 2% Tinuvin 1130 (UVA) and 2% Tinuvin 292 (HALS). All prepared formulations are shown in Table 2.
Sample Preparation
White spruce [Picea glauca (Moench.) Voss] was cut into 57 X 57 x 6 mm samples. The samples were lightly sanded with 80-grit sandpaper and conditioned for 2 weeks at 20 °C/65% RH. Two coats (101-152 µm) of each formulation (A to E) were applied with a foam brush on 24 samples and allowed to dry for 2 weeks. Formulations were also applied on Q-panels for FTIR and glass transition temperature analysis.
Characterization Techniques
Accelerated Aging Tests
The accelerated weathering tests were performed in a Q-sun Xe-3 Xenon test chamber (Q-Lab Corporation, USA) according to ASTM G155.12 The samples were cyclically exposed to full sunlight spectrum at 63 °C on black panel, relative humidity of 50% for 108 min and water spray for 12 min at the same radiation conditions for 2,000 hrs. The irradiance intensity was 0.35 Wm-2.
The color variation before and after accelerated aging was evaluated with a SP60 spectrophotometer from X-Rite following the CIE L*a*b color space. ΔL*, Δa* Δb* and total color change (ΔE*ab) were calculated for each formulation and were compared.
ATR-FTIR Spectroscopy
ATR-FTIR spectroscopy was performed to study the degradation of the various formulations after the aging test. ATR-FTIR spectra were recorded using a Tensor 37 spectrophotometer from Bruker (USA). The surface of the coatings was analyzed by removing the coating with a razor blade from the wood samples. The spectra reported here resulted from the signal between 4,000 and 400 cm-1 averaging 64 scans and nominal resolution of 4 cm-1.
Differential Scanning Calorimetry
Differential scanning calorimeter DCS823e (Mettler Toledo) was used to follow the change in glass transition temperature of formulated nanocomposite coatings before and after accelerated aging. An appropriate amount of dry film of coatings (ca. 15 mg) were sealed in aluminum sample pans. DSC measurements were performed between -60 °C and 100 °C at a heating rate of 20 °C/min.
Results and Discussion
Accelerated Aging
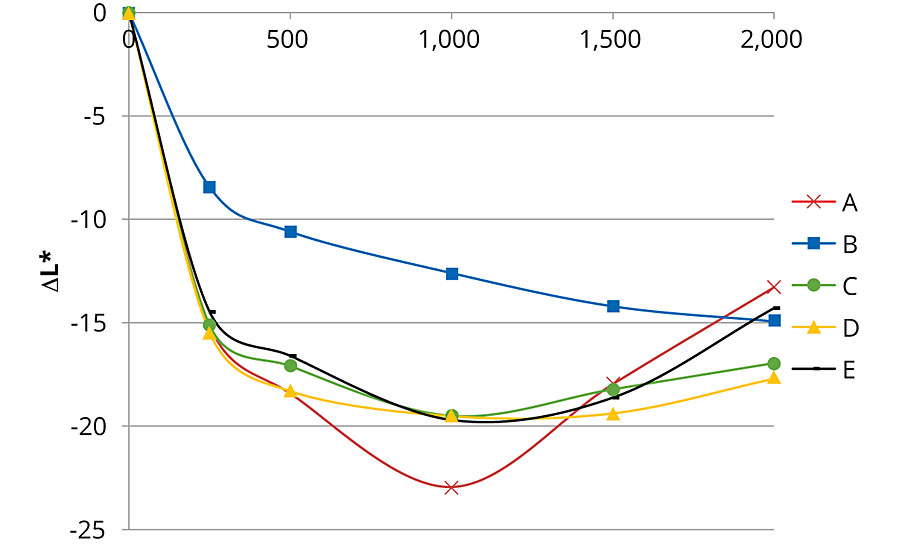
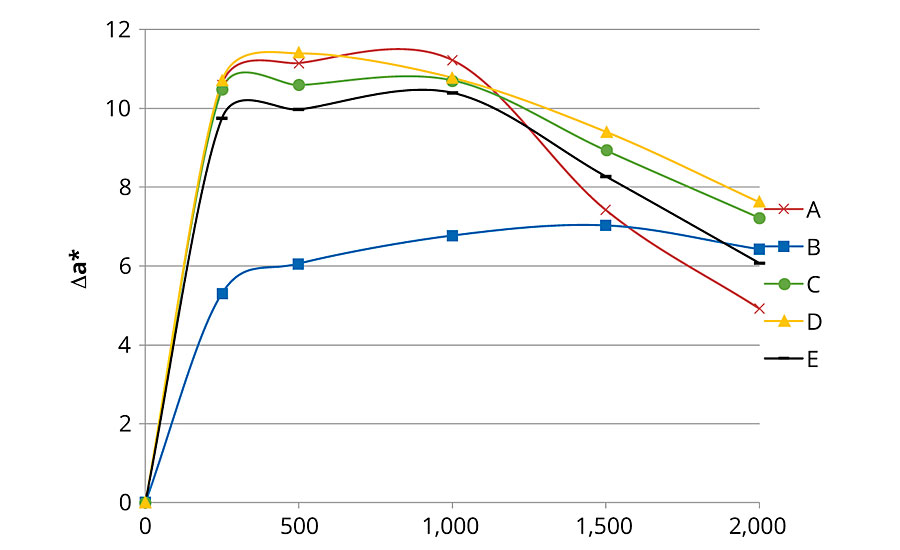
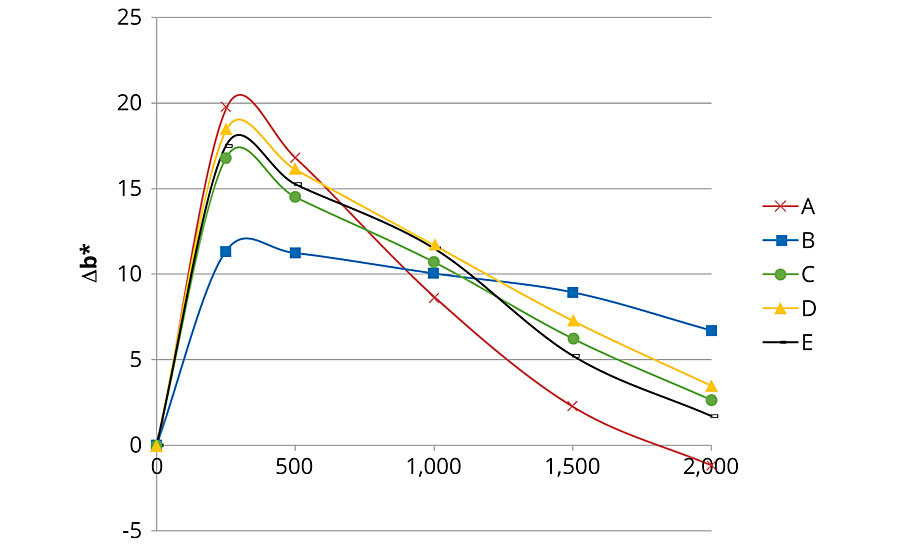
Figures 1, 2 and 3 present color changes in ΔL*, Δa* and Δb* respectively. After 2,000 hrs of accelerated aging, all the formulations tested darkened.The presence of 2% Tinuvin 1130 and 292 slowed down the darkening effect in the first 1,500 hrs. After 2,000 hrs of accelerated aging, formulation E presented a ΔL* similar to formulation B (with Tinuvin 1130 and 292) with a value of -14 and -13 respectively. The presence of Tinuvin 1130 and 292 also slowed down the reddening of the samples for the duration of the test. However, after 2,000 hrs of accelerated aging, all formulations except formulation A presented an Δa* value between 6 and 8. The important increase in yellowing occurring at 300 hrs of UV exposure was due to the formation of photo-stable yellowing compounds resulting from the photo-oxidation of polymeric chains.13 After 500 hrs, all formulations presented a decrease in Δb* values. This phenomenon can be explained by the decomposition of the photo-stable yellowing compounds by visible radiation.14 After 2,000 hrs of accelerated aging, color variation was similar for formulation B (with Tinuvin 1130 and 292) and formulations containing 1% of ZnO nanoparticles (formulation C, D and E).
ATR-FTIR Spectroscopy
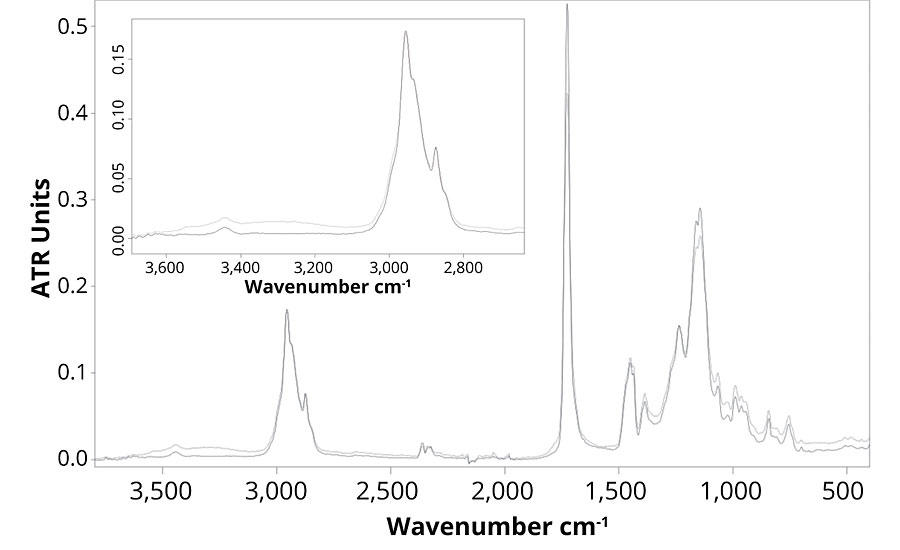
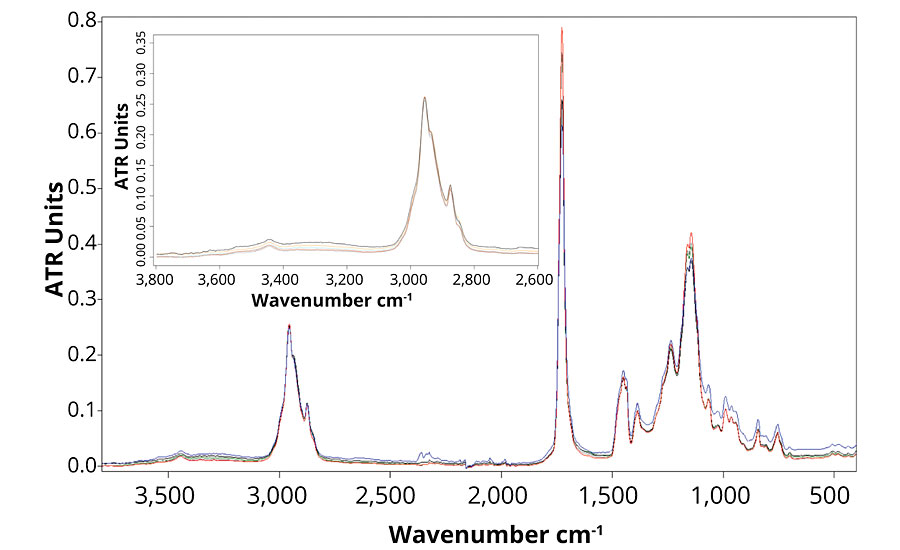
Figure 4 presents the FTIR spectra of formulation B, which contains no UVA or HALS. The FTIR spectra show that the main difference between the weathered and un-weathered samples is detected in the regions of the hydroxyl and carbonyl stretching around, respectively 3,320 and 1,720 cm-1. The small increase in the hydroxyl stretching region shows the presence of oxidized group.15 Also, the small broadening of the carbonyl peak can be explained by the formation of new components following polymer degradation mechanism. Those changes in the FTIR spectra demonstrate that the formulation is subjected to UV degradation, and chemical changes in the polymer structure occur. When observing Figure 5, the same chemical changes are not observed for formulations B, C, D and E, which means that the presence of ZnO nanoparticles prevent photodegradation of the polymer as much as Tinuvin 1130 and 292.
Differential Scanning Calorimetry
Photooxidation of acrylic formulations can be followed by an increase in glass transition temperature (Tg). This increase can be explained by the scission of C-C and C-O bonds that will afterwards recombine with hydrogen or other radicals. This results in an increase in reticulation. Glass transition temperatures of the various formulations before and after weathering are presented in Table 3. As suspected, the addition of ZnO nanoparticles decreased the initial Tg of the formulations, as nanoparticles act as a plasticizer. There was a slight increase in glass transition temperature for all formulations after the accelerated aging test.
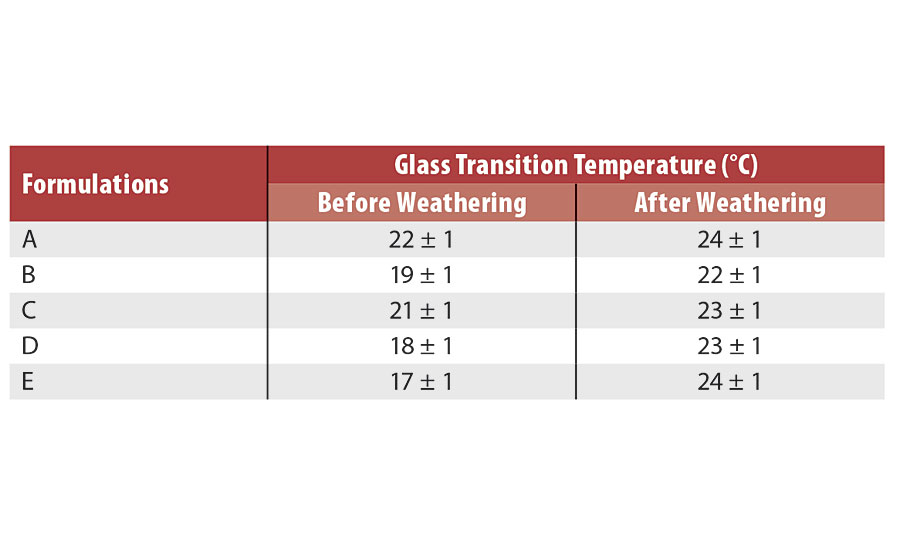
However, it is not possible to conclude if a formulation prevents photodegradation better than another based on Tg measured by DSC. Dynamic mechanical analyses (DMA) are considered as another method to evaluate glass transition temperature and mechanical behavior of the various formulations.
Conclusion
In this work, mini-emulsion polymerization was used to prepare formulations containing various sizes of ZnO nanoparticles in order to increase photostabilization of exterior clear coatings for wood. After 2,000 hrs of accelerated aging, formulations C, D and E presented the same color evolution towards black, red and yellow than formulation B (containing Tinuvin 1130 and Tinuvin 292). FTIR spectra also show that formulations C, D and E presented similar photodegradation as formulation B. This suggests that the use of inorganic UVAs such as ZnO could be effective in photostabilizing exterior clear coatings and the wood underneath. Unfortunately, measurements of Tg by DSC were not conclusive. Dynamic mechanical analyses are considered in further studies. Future work will need to evaluate the dispersion of ZnO nanoparticles into the coating, between the mini-emulsion polymerization and post-addition techniques. Also, longer accelerated aging tests might be necessary to assess if ZnO nanoparticle size affects the photo-stability of the coating.
This paper was presented at the 2019 Waterborne Symposium in New Orleans.
References
1 Stirling, R.; Uzunovic, A.; Morris, P.I. Forest Products Journal 2015, 61, 359-364.
2 Groves, C.K.; McFarling, S.; Gignac, M. Finishing Properties of Canadian Species for Exterior Applications. In Proceeding of the Canadian Wood Preservation Association, 2002; pp 79-104.
3 Evans, P.; Haase, J.; Seman, A.; Kiguchi, M. The Search for Durable Exterior Clear Coatings for Wood. Coatings, 2015. 5(4), 830.
4 Nikolic, M.; Lawther, J.M.; Sanadi, A.R. Use of Nanofillers in Wood Coatings: a Scientific Review. J. Coat. Technol. Res. 2015, 12, 445-461.
5 Weichelt, F.; Emmler, R.; Flyunt, R.; Beyer, E.; Buchmeiser, M.R.; Beyer, M. ZnO-Based UV Nanocomposites for Wood Coatings in Outdoor Applications. Macromol. Mater. Eng. 2010, 295, 130-136.
6 Auclair, N.; Riedl, B.; Blanchard, V.; Blanchet, P. Improvement of Photoprotection of Wood Coatings by Using Inorganic Nanoparticules as Ultraviolet Absorbers. Forest Products Journal 2011, 61, 20-27.
7 Vlad-Cristea, M. S. (2011). Thèse revetement nanocomposites anti UV pour le bois à usage extérieur. PhD, Université Laval.
8 Chimenti, S.; Vega, J.M.; Aguirre, M.; Garcia-Lecina, E.; Diéz, J.A.; Grande, H.-J.; Paulis, M.; Leiza, J. R. Effective Incorporation of ZnO Nanoparticles by Mini-Emulsion Polymerization in Waterborne Binders for Steel Corrosion Protection. J. Coat. Technol. Res. 2017, 14, 829-839.
9 Landfester, K.; Weiss, C.K. Encapsulation by Mini-Emulsion Polymerization. In: Caruso, F (ed.) Modern Techniques for Nano- and Microreactors/-Reactions, p.. 1-49. Springer, Berlin (2010).
10 Boivin, G.; Ritcey, A.; Morris, P.I.; Landry, V. Black Stain-Resistant Acrylic Latexes for Wood Coatings Applications. In Proceeding of the International Research Group on Wood Protection the 48th Annual Meeting, Ghent, Belgique, 2017; Document no. IRG/WP 17-40786.
11 Mikulski , J.; Sikora, B.; Fronc, K.; Aleshkevych, P.; Kret, S.; Suffczynski, J.; Papierska, J.; Klopotowski,L.; Kossut, J. RSC Adv. 2016, 6, 44820-44825.
12 ASTM G 155-00. Laboratory Apparatus; Degradation of Materials. “Practice for Operating Xenon-Arc Light Apparatus for Exposure on Nonmetallic Materials”, Annual Book of Standards, Section 14, vol. 14.04, 2000 (Easton, MD ASTM, 2000).
13 Queant, C.; Landry, V.; Blanchet, P.; Schorr, D. Synthesis and Incorporation of Poly (Methyl Methacrylate) Microspheres with UV Stabilizers in Wood Clear Coating Binder. Journal of Coatings Technology and Research 2017, 1-12.
14 Lemaire, J. (1996). Predicting Polymer Durability. Chemtech: 42-47.
15 Chiantore, O.; Trossarelli, M.; Lazzari, M. Photooxidative Degradation of Acrylic and Methacrylic Polymers. Polymer, 2000, 41, 1657-1668.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









