Liquid EPDM for Waterborne Coatings

Ethylene-Propylene Diene Monomer (EPDM) rubber has been used extensively in automotive, wire and cable, roof membrane, and thermoplastic vulcanite (TPV) applications due to the ease of processing imparted by the polymer’s unique structure.1,2 A crosslinking process is usually required to achieve the desired chemical and physical properties, and is accomplished through the incorporation of sulfur or peroxide, and the application of heat and pressure to activate the crosslinking agents and accelerators. However, in some cases, it can be difficult or unfavorable to apply heat to initiate crosslinking, and a low-temperature reaction is preferred.
Coatings, adhesives and sealants are one such application in that the rubber is applied in a thin film over a large area, making it difficult to heat the elastomer for curing. EPDM has excellent water, vapor, chemical and electrical barrier properties that greatly improve on elastomeric acrylic, polyurethane and silicone coatings currently available. These properties make EPDM ideally suited for roof coatings and anti-corrosion coatings, especially in low-temperature or high-humidity environments. However, an ideal combination of proper application viscosity, room temperature crosslinking and sufficient working time must be achieved for practical utility.
If we look at the EPDM rubber industry, we find many studies demonstrating room-temperature crosslinking. Photocuring has been demonstrated with several photoinitiators, but the requirement of a very strong ultraviolet (UV) source limits the applications in which it can be used.3 Peroxides have also been used, but these still typically have 10-hour half-life temperatures in excess of 50 °C, must be mixed immediately prior to application and have very short application times, making them unfavorable for coatings.4
In a recent study, two coating formulations from a commercially available low-molecular-weight EPDM were demonstrated using a peroxide crosslinking mechanism and a sunshine UV crosslinking mechanism.5,6 Both formulations maintained good EPDM rubber properties after crosslinking, including high mechanical strength, low-temperature flexibility, hydrophobicity and adhesion to polar substrates. The formulations met industrial requirements as protective coatings in solid content, rheology, curing speed, shelf life and pot life, and can be produced at a cost close to that of commercial elastomeric acrylic coatings. These coatings were also formulated to be used as liquid roofing membranes and showed improved performance over solid EPDM sheets and elastomeric acrylic coatings in terms of ease of installation, solar reflectance, water resistance and weather durability.
In this article, we report a waterborne dispersion of liquid EPDM that may be used for coatings and adhesives. The path to formulating a peroxide-cured, sunshine UV-cured, and an oxidatively cured system are discussed. While the mechanical properties are lower than that of their solvent-based counterparts, these low-volatile organic compound (VOC) coatings retain low-temperature flexibility, hydrophobicity, excellent adhesion and low water vapor transmission properties. Blending with elastomeric acrylic latexes is also presented.
Experimental
Waterborne Dispersion of L-EPDM
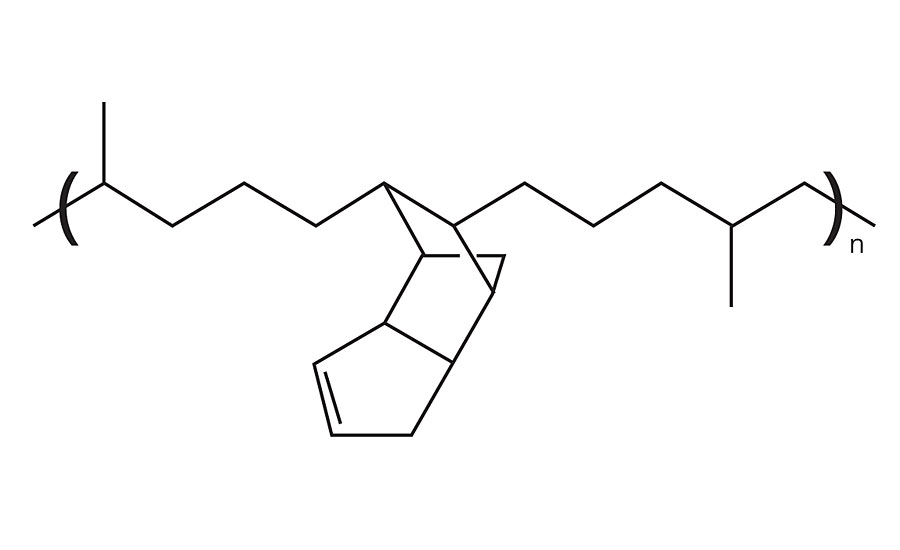
The L-EPDM used in this study was a Trilene® T65 with 10.5% dicyclopentadiene (DCPD) and ethylene/propylene ratio at 50/50. A simplified structure is shown in Figure 1. The molecular weight of the EPDM polymer was determined from dynamic viscosity measurement to be around 50,000 Dalton, much lower than that of typical EPDM polymers used for bulk compounding applications. This L-EPDM was dispersed in water with a proprietary surfactant using a method described in the patent literature.7
Coating Formulation and Testing
Table 1 lists the formulations used in this study. Formulation A employs a peroxide crosslinking system in the presence of cobalt and zirconium catalysts. Formulation B omits the peroxide. Formulation C used a photoinitiator as the crosslinking agent. Sources of materials are also listed. All raw materials were used without being further purified.
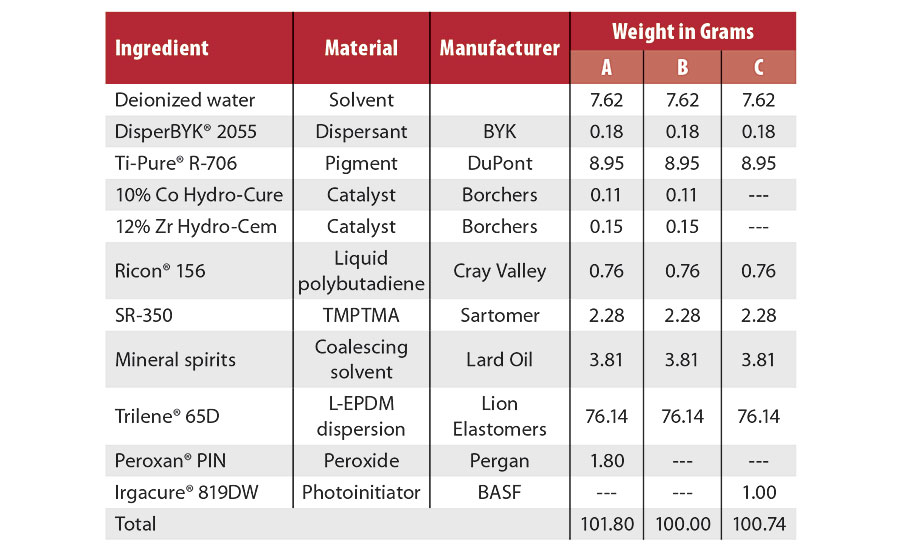
In all formulations, the dispersant was dissolved in water before adding the pigment. The blend was then mixed at 2,000 rpm in a FlackTek SpeedMixer™ until a homogenous dispersion was formed. Catalysts, if used, were then added to the pigment dispersion and mixed for a further 2 min. Ricon 156, SR-350 and mineral spirits were blended in a scintillation vial until fully dissolved, added to the L-EPDM polymer dispersion, and then mixed at 2,000 rpm in the FlackTek SpeedMixer to achieve a uniform blend. The pigment blend and polymer dispersion were then blended at 1,000 rpm to form the coating. In formulation C, the photoinitiator was added as the final mixing step. Formulation A employed the peroxide curative (used as received) as a separate component to avoid premature crosslinking. The coatings were evaluated for their specific gravity, solid content and viscosity following ASTM standards with standard lab equipment.
The coating was applied to three different substrates - steel, glass and release paper - using a Gardco wet film applicator to 0.5-1.0 mm thickness. Formulation A required blending part A and part B before application. Coatings of formulations A and B were dried indoors at room temperature for 7 days. Formulation C was dried outdoors with typical sunshine for 2 days, and allowed to rest indoors for a further 5 days before testing. During the drying process, the dry time was measured following ASTM D1640 using a standard dry time tester. After drying, the coating was evaluated for physical properties including tensile strength, elongation-at-break, pencil hardness, crosshatch adhesion, impact strength and moisture vapor transmission resistance (MVTR) following related ASTM standards with standard lab equipment.
Blending with Elastomeric Acrylic Resins
Formulation B was blended with UCAR® 3176 A and Rhoplex® 2100-EC from Dow Chemical at levels from 5-50 wt%. All samples were mixed at 2,000 rpm in a FlackTek high-speed mixer until a homogenous dispersion was formed. The coating was applied to release paper using a Gardco wet film applicator to 0.5-1.0 mm thickness. Samples were observed for substrate wetting and tested for percent elongation and tensile strength.
Results and Discussion
Properties of Waterborne Dispersion of L‐EPDM
This dispersion is a milky, white liquid (48 wt% solids, pH 8.0, particle size 1-2 microns, density 0.9 g/mL and viscosity ~ 14,000 cP as measured via Brookfield viscometer, spindle 64, 20 rpm, 20 °C). This dispersion is stable from separation for more than one year. If separation does occur, the L-EPDM particles float and leave a thin layer of water at the bottom of the container. The dispersion can be re-homogenized by shaking or stirring.
Properties of Coatings Formulations
Table 2 lists the properties of wet paint and dry coating films of the three formulations described above.
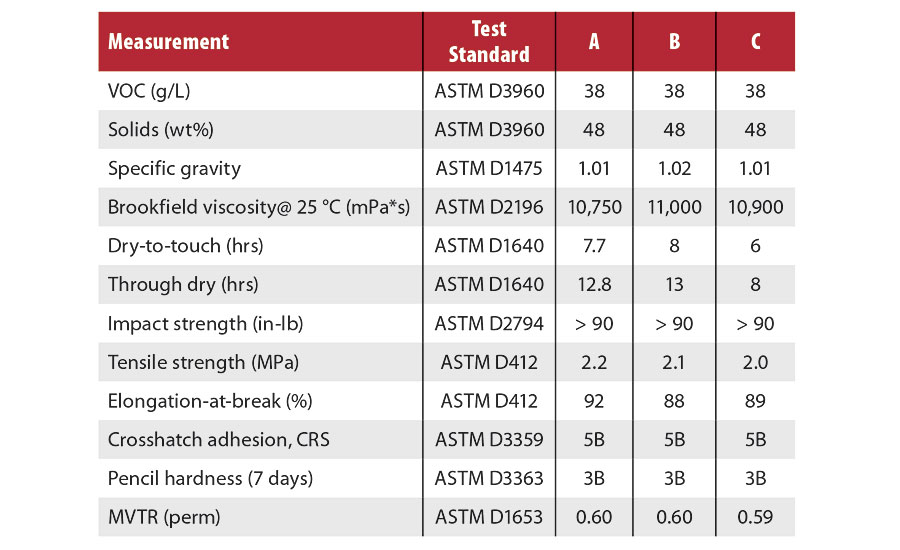
The viscosity of the original dispersion is low enough to allow for pumping and easy mixing of the formulations. The added water of the pigment dispersion further lowers the viscosity, allowing these coatings to easily be applied with the same methods typically used for paints, including rolling, brushing and spraying. After curing, all formulations display good mechanical strength and elongation-at-break, indicating sufficient degree of crosslinking, as needed in an elastomeric material. Notably, all formulations have excellent adhesion to steel substrates and a high moisture vapor transmission rate (MVTR), making them excellent for corrosion protection.
It is also notable that the inclusion of peroxide in the formulation only moderately decreases the dry time of the coating, and has a minor effect on the physical properties of the final film. Thus, it is unnecessary to add the extra expense and mixing step of the peroxide when making this coating. The differences in mechanical properties can be made up through formulation changes and are the focus of current work.
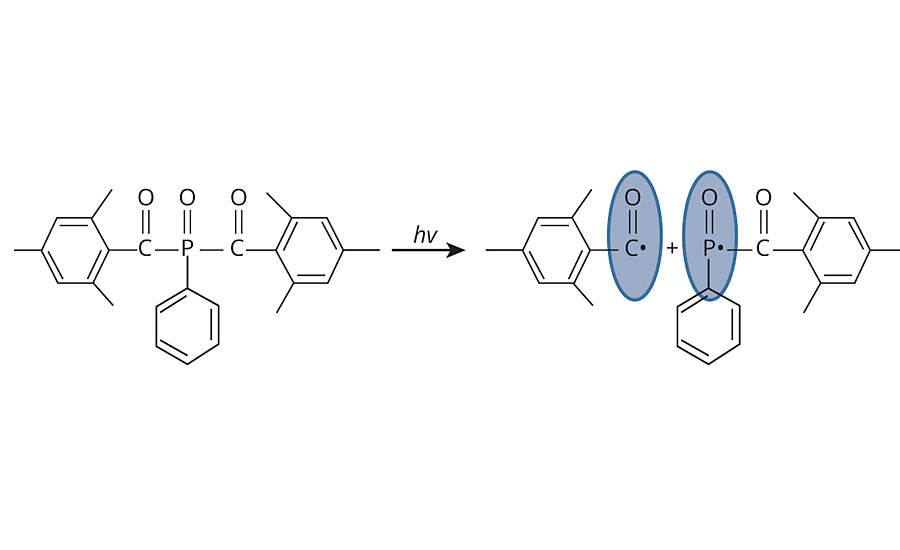
The efficiency of the photocrosslinking of the waterborne L-EPDM coating is interesting. The choice of water-dispersible Irgacure 819DW is key to the success of this coating. The bisacylphosphine oxide (BAPO) type of photoinitiator is able to cure the EPDM polymer efficiently as it dissociates under the influence of the UVA wavelength (315-400 nm) that is common in natural sunshine (Figure 2). BAPO is also excellent at both surface and through cure, and is not inhibited by TiO2 pigment.
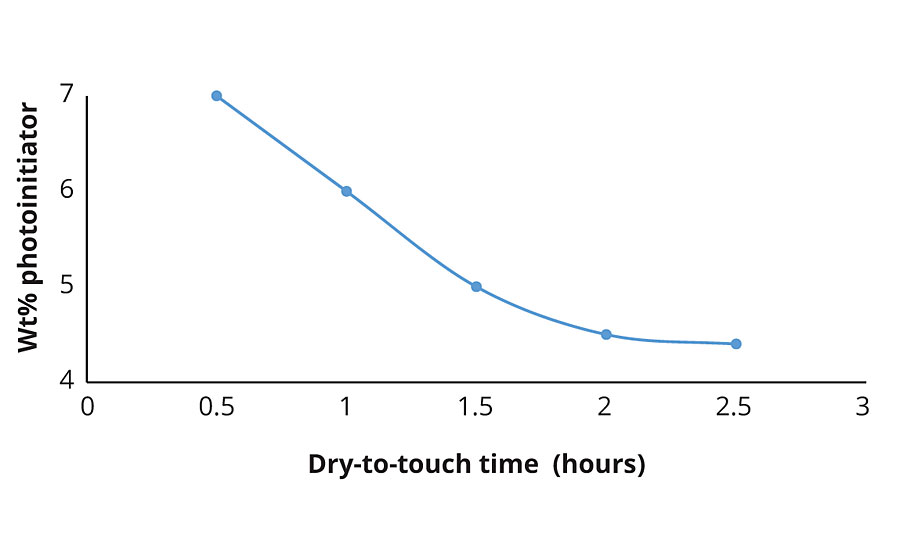
The loading level of the photoinitiator also affects the cure rate of the coating. Figure 3 shows that there is a limit to the amount of photoinitiator required to achieve optimal cure time. If the cure is too rapid, the applicators will not have sufficient time to properly cover the substrate. Thus, it is unnecessary to use greater than 4.5 wt% of the photoinitiator. The effect of photoinitiator loading on mechanical properties is the subject of an ongoing study.
Properties of Waterborne L-EPDM Blends with Elastomeric Acrylic Latexes
Pure samples of both the UCAR and Rhoplex elastomeric acrylic latices had poor wetting on the release paper and beaded upon application. With increasing L-EPDM content, the substrate wetting improved, and at 50 wt% loading of the L-EPDM formulation, the wetting was as good as that of the L-EPDM formulation itself. Tensile strength increased and percent elongation decreased with increased L-EPDM loading (Figure 4), indicating the formation of an interpenetrating network at this loading. The improved wetting and tensile strength indicate the utility of L-EPDM as a reinforcing additive for elastomeric roof coatings. These results are similar to latex blends previously prepared by Lion.

Conclusions
Despite the challenges of reformulating a coating from solvent-based to waterborne and curing a low-molecular-weight EPDM at ambient temperature, formulations have been successfully developed utilizing peroxide, oxidative and UV-curing systems. These systems meet the application standards of viscosity, cure rate, mechanical strength, and adhesion. These discoveries also lead to potential applications in adhesives and sealants. The systems described in this study demonstrate the utility of L-EPDM in waterproof roof and industrial coatings.
References
1 Karpeles, R.; Grossi, A.V. EPDM Rubber Technology, Handbook of Elastomers, 2nd Ed., New York, 2001, 845.
2 Riedel, J.A.; Laan, R.V. Ethylene Propylene Rubbers, The Vanderbilt Rubber Handbook, 13th Ed., Norwalk, CT, 1990, 123.
3 Hilborn, J.; Ranby, B. Rubber Chemistry and Technology, 1988, 61, 568.
4 Dluzneski, P.R. Rubber Chemistry and Technology, 2001, 74, 451.
5 Molnar, M.J.; Nelson, D.S.; Fontenot, A.J.; Young, H.W.; Ibay, J.A.; Zhu, Z. U.S. Patent 20140228469 A1, 2014.
6 Molnar, M.J.; Fontenot, A.J.; Young, H.W.; Nelson, D.S.; Ibay, J.A. U.S. Patent 20140228506 A1, 2014.
7 Zhu, Z. U.S. Patent 9,321,915 B2, 2016.
For more information, email greg.brust@lionelastomers.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









