Intelligent Binders - A Cloak of Invisibility for Ships

Maritime transport is the first preference for transporting cargo; more than 50,000 ships navigate the world’s oceans, carrying nearly 90% of all goods transported worldwide. The commercial viability of the global merchant fleet depends heavily on the shell of the vessels. Biofouling, caused by plant and animal growth on the hull below the waterline, is a particular concern. Proteins, carbohydrates and other micronutrients from the ocean begin adhering themselves to the hull upon its initial contact with seawater. As nutrients, they attract marine organisms that then settle onto the shell. The biofilms formed in this process are highly complex habitats in which several hundred species interact (positively or negatively) with one another, thus hindering the movement of the ship (Figure 1).

FIGURE 1 » How biofilms form – Just seconds after contact with seawater, micro-organisms settle on the ship’s hull. These serve as nourishment for other organisms, and a biofilm is formed.
The significant roughening of the hull’s surface due to biofouling leads to a drastic increase in frictional resistance during movement. The result is a vicious cycle: to maintain the same speed of travel, the ship needs more energy and fuel, with an associated rise in CO2 emissions. In addition, the maneuverability of the ships is reduced and the risk of corrosion increased. Hence, ships need more frequent stays in the dry dock for cleaning and repairs. Shorter maintenance cycles and increased fuel consumption result in higher costs for shipping companies.
Demand is therefore high for coatings that protect ships against biofouling. So far, the most effective method for protecting the hull is biocide-containing antifouling coatings. Their functioning is based on a leaching process in which active toxic substances are dissolved out of the coating matrix and migrate to the surface of the ship’s hull. Here, they form a kind of biocide cloud around the ship and damage micro-organisms before they settle on the body. In the past, very effective biocides like tributyltin hydride (TBT) were used. However, investigations have revealed that the toxicity of the biocides is not limited to organisms at the hull’s surface; it also extends to other species in the sea. These organisms then face conditions that may lead to their extinction. As a result of this finding, TBT was banned for use in antifouling paints worldwide in 2008. The active agent of choice today is copper oxide, which is regarded as more eco-friendly than TBT and acts on the same leaching principle. But it, too, is a heavy metal oxide, and the copper ions dissolving out from the coating can be toxic in high concentrations. Hence, biocidal coatings are becoming increasingly regulated, creating a demand for high-performing, nontoxic alternatives.
Foul-Release Concepts and Test Methods
Most biocide-free foul-release coatings on the market provide an easy-to-clean surface. The formulations usually include hydrophobic binders, like silicone hybrid resins, sometimes infused with silicone oil or fluorinated compounds that move to the coating’s surface. In static conditions, e.g. while the ship is loaded or lying in the roads, organisms adapted to hydrophobic environments settle on these coatings because no biocide is used to kill them. However, due to the surface’s usually low surface energy and high elasticity, the adhesion strength of the organisms is low. While the vessel is moving, in dynamic conditions, the shear forces are strong enough to detach the biofouling film and clean the ship’s hull.1 On the other hand, it is known that hydrophilic polyethylene glycol (PEG) can act as a repellant against micro-organisms and cell adhesion. This is rooted in its hydration strength and high configurational mobility.2 While both concepts have shown promising effects against biofouling, recent work has focused on the combination of hydrophilic and hydrophobic moieties.3
Our own chemical approach started with SILIKOPON® EF, a commercially available silicone epoxy hybrid binder, well known for its anti-adhesive properties and its chemical and mechanical resistance. This mainly hydrophobic material was modified with hydrophilic moieties, leading to an amphiphilic binder system (Figure 2).
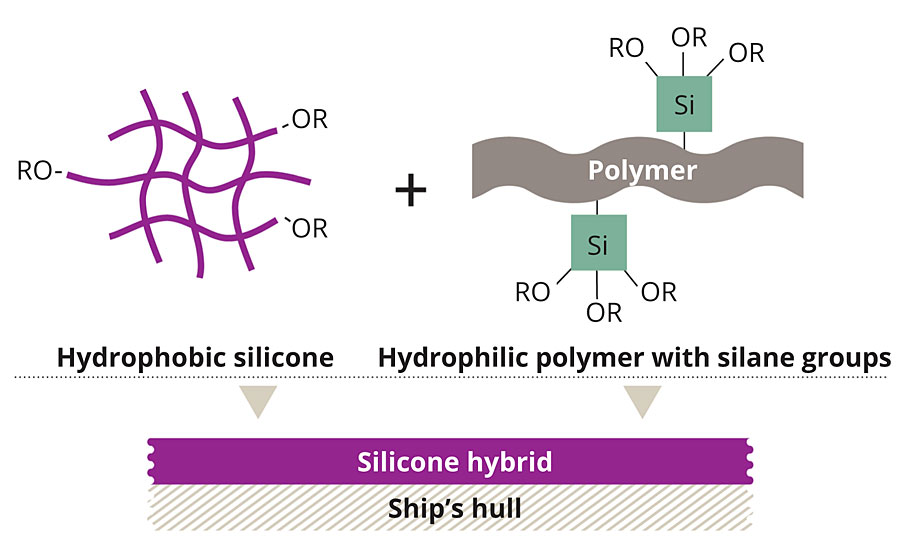
FIGURE 2 » Uniting the benefits – Using a curing catalyst, hydrophobic silicone was combined with a hydrophilic polymer.
These polymers can be cured to smooth surfaces using a dual-cure mechanism consisting of an epoxy ring-opening reaction and hydrolysis-condensation reaction. During this process and upon immersion in water, the hydrophilic moieties orient towards the coating surface, creating a hydrate layer. When fully formulated and applied to a ship, this hydrate layer masks the vessel’s hull, as if it was water. The aquatic organisms cannot differentiate the ship’s surface from the surrounding seawater, and thus they refrain from settling. A natural principle is exploited here, as living beings always seek out the most favorable habitat for reproduction (Figure 3).
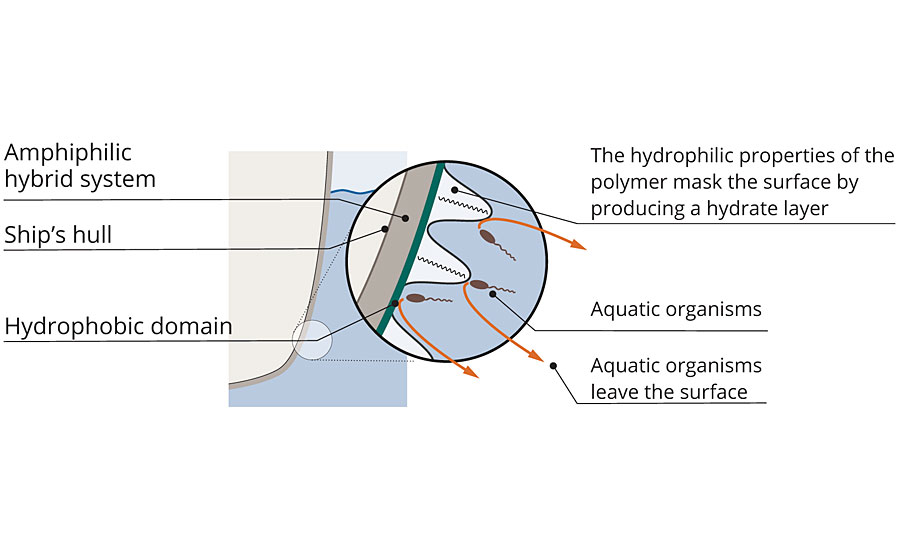
FIGURE 3 » Protection against biofouling – In the new system, hydrophobic and hydrophilic domains alternate. Aquatic organisms can no longer clearly recognize the surface.
Over the course of the development work, it was necessary to test the fouling release performance of the newly established binder system. The most environmentally relevant test method is seawater exposure. Aside from the high preparatory effort, which makes it unsuitable for screening purposes, the method underlies seasonable growth conditions. Especially in cold waters like the North Sea, these differences hinder continuous testing. To get fast and seasonally independent results, we sought new methods for biofouling evaluation. In cooperation with the University of Muenster (Germany), a three-step approach was developed, mimicking marine biofouling on ships with increasing biological complexity. For this, microcosm and mesocosm studies were combined with sea trials.
The first steps in testing antifouling coatings typically entail quantifying biofilms formed by diatom or bacterial mono-cultures, e.g. by staining nucleic acids with fluorescent dyes4 or by spectrophotometric or fluorimetric quantification of chlorophyll.5 Although this is not so environmentally relevant (because the natural communities are much more complex), it was decided to install a photoautotrophic co-culture microcosm as a first step in this study. For the micro-fouling test system with defined cultures, the photoautotrophic diatoms Amphora coffeaeformis or Phaeodactylum tricornutum with the heterotrophic bacterium Alteromonas macleodii were chosen. All three species are amongst the first settlers on a newly immersed surface. While A. coffeaeformis prefers hydrophobic surface areas,6 the settling of P. tricornutum is more pronounced on hydrophilic surfaces.7,8
To fill the gap between microcosm experiments and sea trials, a mesocosm system was developed. An acrylic glass aquarium with two adjacent basins was designed. The basins were filled with seawater and inoculated with biomass originating from biofilm-covered stones from the North Sea (Figure 4).
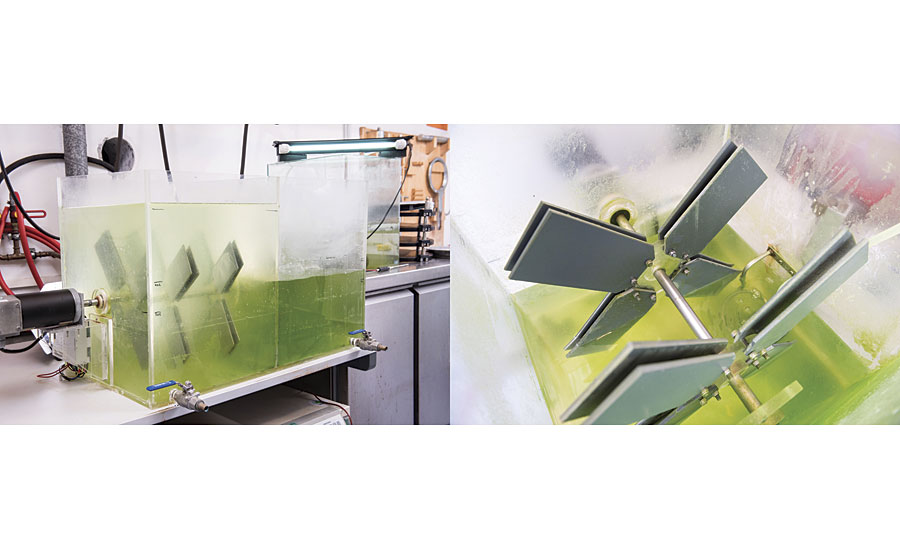
FIGURE 4 » Aquarium system used for mesocosm experiments. (A) Complete view of the aquarium system including the motor unit and the two separated basins; the grey PVC panels (20 cm length) carry the different coatings on one side. (B) Detailed view on the coated plates mounted on the plate holders with self-retaining nuts.
The conditions and nutrients were chosen to ensure growth of photoautotrophic algae and heterotrophic bacteria. The aeration of the aquarium caused slight water movement along the coated PVC plates supporting growth of the attaching species, but did not apply shear forces to the surfaces. Based on macroscopic visual inspection, each inoculation of the mesocosm led to similar algal blooms in the liquid phase and similar biofilm formation on the uncoated PVC control plates. The mounted PVC plates were incubated for five weeks at 21 °C, with 16 hrs illumination per day (static growth conditions). After that, the plates were rotated at 60 rotations per minute for one week (dynamic conditions), before being dismounted and evaluated. During rotation, the outer parts of the plates were moving at 2.45 knots. Towards the center of the plates, the circular velocity was decreasing with the radius, thereby creating a shear force gradient along the biofilms. This relatively slow rotation was chosen to identify coatings showing fouling-release properties already at low shear forces. Such coatings are likely to exhibit effective foul release performance in the sea trials.8
Finally, sea trials were performed in the North Sea in Hooksiel, Germany. This is a marine site with cold water and seasonal growth behavior. The coated PVC panels were exposed under static conditions from March to August 2017. The plates were visually inspected every 15 days to monitor the degree of biofouling. In addition, a certain area of the fouled plates was wiped with a silicone doctor blade in a defined way as surrogate for shear forces to assess how firmly the biomass was attached to the individual coatings. According to pre-experiments, this mechanical treatment induces foul release effects comparable to treatment with a water jet at 200 bar.
Results and Discussion
To evaluate the foul release properties of the newly developed amphiphilic binders, three compositions S1 to S3 were chosen with varying hydrophilic-hydrophobic balance. The newly prepared binders were mixed with DYNASYLAN® AMEO and 2% dioctyltin dicarboxylate as catalyst, applied by either film applicator or air spray at 300 µm wet film thickness onto PVC sheets and glass slides and cured at room temperature for at least 24 hrs until hard dry. As a comparison, commercial foul release paint C1 was included in the study (Table 1).
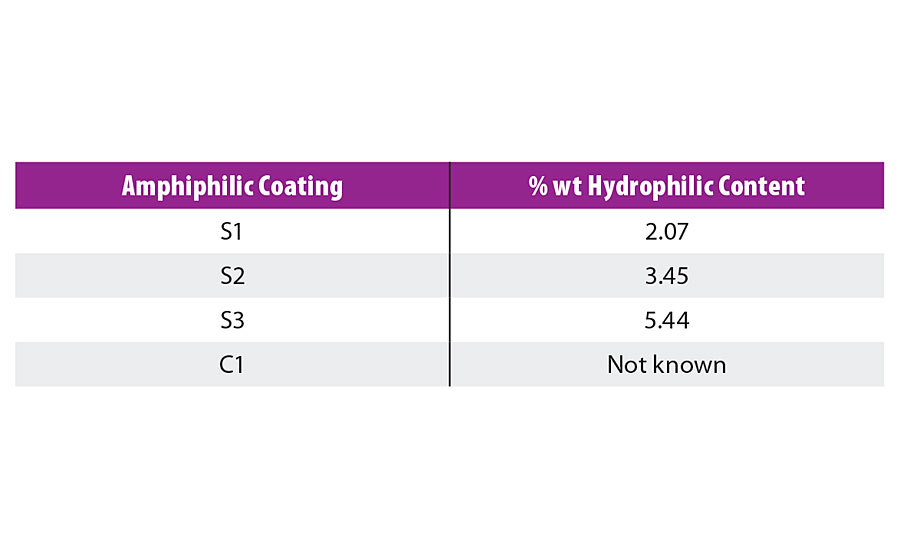
TABLE 1 » Composition of the amphiphilic coatings with their hydrophilic content.
To ensure that the foul-release properties of the newly developed system were not caused by the leaching of toxic compounds, toxicity assays with conditioned media were performed. To test the effects of the conditioned media on bacterial physiology, a bioluminescent reporter strain of the common marine bacterium A. macleodii was used. Growth inhibition as well as cell lysing properties were investigated. It was shown that none of the films S1 to S3, nor C1 exhibited any toxic properties (see Reference 8 for details).
In the next step, the antifouling properties of the different binders on glass slides were tested in microcosm co-cultures of the wild type of A. macleodii with P. tricornutum or A. coffeaeformis, respectively, for two weeks under photoautotrophic growth conditions by static immersion. Chlorophyll fluorescence was used to quantify the biofilm growth. A washing procedure was installed, which served as a surrogate for applying shear forces caused by water movement of a fast-moving ship. The incubation of the glass slides was repeated twice by exposing the same plates to fresh co-cultures. The repetitions should account for potential microbial modifications of the surface, which may lead to altered fouling-release properties. The quantification of chlorophyll fluorescence was restricted to the central area of the coating and normalized to the quantified area. In the following, the normalized diatom biomass is shown for easier comparison. The normalized diatom biomass was calculated by dividing the chlorophyll fluorescence for each coating replicate by the mean of the respective uncoated control. Normalized diatom biomass below 1 indicates fouling-release properties (Figure 5).
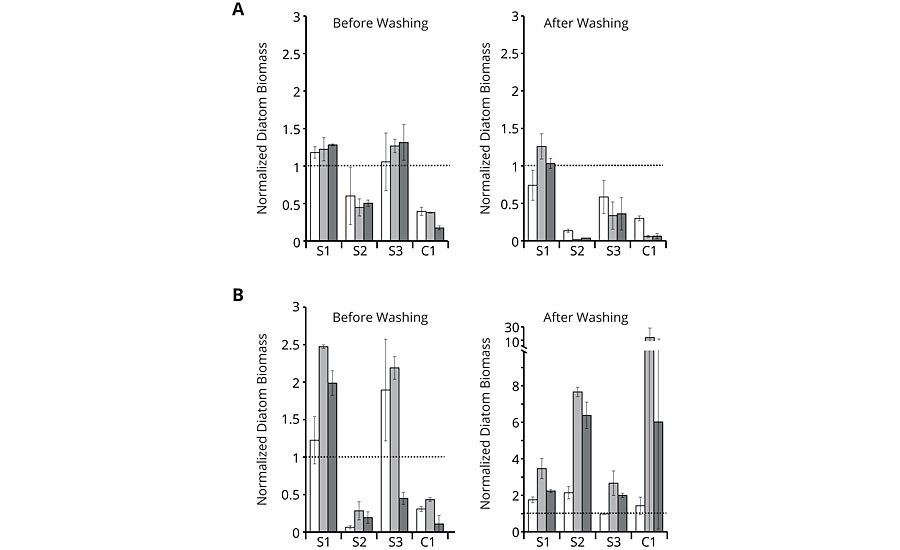
FIGURE 5 » » Growth experiments with co-culture of the diatoms P. tricornutum (A) or A. coffeaeformis (B) with the wild type of the bacterium A. macleodii. Normalized diatom biomass based on chlorophyll fluorescence of the first (white bars), second (light grey bars) and third (dark grey bars) repetition is shown for amphiphilic binders S1 to S3 and commercial coating C1. Chlorophyll fluorescence was measured before and after washing for each repetition. All values significantly below 1 indicate less fouling than the plain glass reference (dashed line). Error bars indicate standard deviation (n=3).
While the results before washing demonstrate mere organism attachment, the results after washing reveal true adhesion of the species. As can be seen from the graphs, amphiphilic binder S2 showed low orders of attachment before washing with both co-cultures (A) and (B) comparable to the commercial reference C1. After washing, S2 and C1 still showed good results with co-culture (A), but delivered high normalized fluorescence values with co-culture (B), indicating strong adhesion of the few attached species. S1 and S3 exhibited mostly high orders of attachment and adhesion with both co-cultures. Although S1 to S3 have a linearly increasing content in hydrophilic moieties, the microcosm assays did not directly react to this. The most hydrophilic binder S3 had lower adhesion of co-culture (A) compared to the most hydrophobic variant S1, even though co-culture (A) included the diatom P. tricornutum, which prefers hydrophilic surfaces for its settlement. It seems that the hydrophilic surface structure does not necessarily follow the hydrophilic content. These findings underline the complexity of the interaction between the biological and the chemical system, making it hard to predict good foul release performance without biological pre-screening.
For the mesocosm experiments, coated PVC sheets were immersed in natural seawater in which an algal bloom had been artificially induced. Water and biomass originated from the North Sea to keep this system as close to the actual sea trials as possible. Binders S1 to S3 and commercial reference C1 were exposed for five weeks under static and one week under dynamic conditions, before they were dismounted and evaluated. Dismounting the plates before the endpoint of the investigation was not possible without damaging the attached biofilm, thereby deteriorating the test results. Macroscopic visual inspection at the endpoint showed that the amphiphilic binders S2 and S3 were scarcely fouled, whereas S1 and C1 were nearly completely covered by a biofilm (Figure 6, upper row). Visualization of the chlorophyll fluorescence on all coatings showed a very similar pattern as the macroscopic visual inspection indicating that the biofilms contained photosynthetic microorganisms (Figure 6, lower row and graph). For the quantification of the photosynthetic biomass, the normalized photosynthetic biomass was calculated by dividing the chlorophyll fluorescence on the individual coatings by the chlorophyll fluorescence of the respective controls. Values below 1 represent foul release properties. Chlorophyll fluorescence of the coated and uncoated PVC plates prior to incubation was negligible (data not shown). Summarizing the mesocosm studies, the replicates of the individual coatings were similarly fouled, as pointed out by the mainly low standard deviations. This indicates that the fouling did not occur fortuitously, but it was apparently governed by the specific surface properties of the coatings. Again S2 was one of the best performing binders, outperforming the commercially available reference C1.
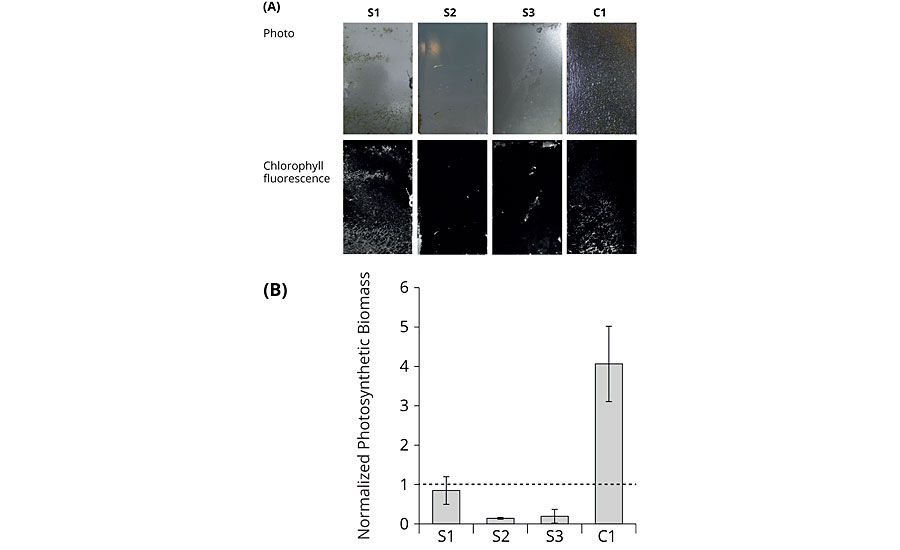
FIGURE 6 » End-point analysis of mesocosm growth experiments. (A) Photographs of the coated plates for macroscopic visual inspection (upper row); visualization of chlorophyll fluorescence of the coated plates (lower row); one representative out of three replicates for each coating was selected for the figure. The circular velocity decreases from top to bottom. Water flow was directed from the right side. (B) Normalized photosynthetic biomass based on chlorophyll fluorescence. Error bars indicate standard deviation (n=3).
Sea trials were conducted in the North Sea in Hooksiel, Germany, from March to August 2017 by exposing the amphiphilic binders S1 to S3 and the commercial reference C1 on PVC plates to the natural organism community by static immersion. To evaluate the foul-release performance, static immersion is not very suitable. Therefore, a wiping procedure was included in the visual inspection, simulating a fast-moving ship or a water jet cleaning. On all coatings, macro-fouling developed during the exposure. After three months of incubation, the attached biomass could be wiped off from all coatings except S1, on which a thinner layer of biomass remained. After five months of incubation, biomass could only be wiped off from S2. Also the commercial reference C1 was overgrown with a strongly attached biomass. In summary, S2 appeared to be the best foul release coating in the test, performing better than commercially available coating C1 (Figure 7).
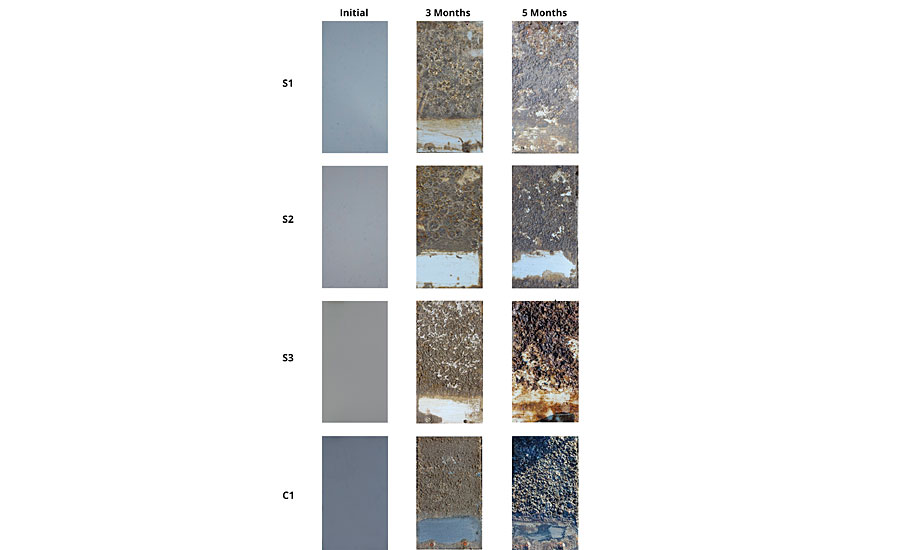
FIGURE 7 » Photographs of the coated PVC panels before and after seawater exposure. The lower part of the panel was wiped with a silicone doctor blade during every inspection.
Conclusion
In conclusion, new amphiphilic silicon epoxy hybrid binders were developed for biocide-free foul-release coatings. In their mode of action, the hydrophilic parts of these binders will form a hydrate layer on the surface of the ship’s hull, leading to delayed recognition by marine organisms. The hydrophobic parts, on the other hand, will impose an easy-to-clean effect to the surface, leading to good foul release properties under dynamic conditions.
The new binders were tested in a multistep approach, starting from microcosms over a mesocosm to static seawater exposures. This methodological approach opens the opportunity for fast screening of binder properties in seasonally independent tests. Neither microcosm nor mesocosm can substitute for real seawater exposures. However, they can give a good indication towards the foul-release properties in real-life situations.
References
1 Beigbeder, A.; Degee, P.; Conlan, S.L.; Mutton, R.J.; Clare, A.S.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Dubois, P. Biofouling 2008, 24, 291-302. Wynne, K.J.; Swain, G.W.; Fox, R.B.; Bullock, S.; Ulik, J. Biofouling 2000, 16, 277-288. Brady, R.F.; Singer, I.L. Biofouling 2000, 15, 73-81.
2 Prime, K.L.; Whitesides, G.M. J. Am. Chem. Soc. 1993, 115, 10714-10721. Ma, H.; Hyun, J.; Stiller, P.; Chilkoti, A. Adv. Mater. 2004, 16, 338-341. Schilp, S.; Kueller, A.; Rosenhahn, A.; Grunze, M.; Pettitt, M.E.; Callow, M.E.; Callow, J.A. Biointerphases 2007, 2, 143-150.
3 Galhenage, T.P.; Webster, D.C.; Moreira, A.M.S.; Burgett, R.J.; Staslien, S.J.; Vanderwal, L.; Finlay, J.A.; Franco, S.C.; Clare, A.S. J. Coat. Technol. Res. 2017, 14, 307-322. Hawkins, M.L.; Schott, S.S.; Grigoryan, B.; Rufin, M.A.; Ngo, B.K.D.; Vanderwal, L.; Stafslien, S.J.; Grunlan, M.A. Polym. Chem. 2017, 8, 5239-5251.
4 Vesterlund, S.; Paltta, J.; Karp, M.; Ouwehand, A.C. J. Microbiol. Methods 2005, 60, 225-233.
5 Galhenage, T.P.; Webster, D.C.; Moreira, A.M.S.; Burgett, R.J.; Staslien, S.J.; Vanderwal, L.; Finlay, J.A.; Franco, S.C.; Clare, A.S. J. Coat. Technol. Res. 2017, 14, 307-322. Sommer, S.; Ekin, A.; Webster, D.C.; Stafslien, S.J.; Daniels, J.; van der Wal, L.J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A. Biofouling 2010, 26, 961-972. Zhou, T.; Calabrese, D.R.; Taylor, W.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Fischer, D.; Kramer, E.J.; Ober, C.K. Biofouling 2014, 30, 589-604.
6 Cassè, F.; Swain, G.W. Int. Biodeterior. Biodegrad. 2006, 57, 179-185. Zargiel, K.A.; Swain, G.W. Biofouling 2014, 30, 115-129.
7 Buhmann, M.T.; Schulze, B.; Förderer, A.; Schleheck, D.; Kroth, P.G. J. Phycol. 2016, 52, 463-474. Dugdale, T.M.; Willis, A.; Wetherbee, R. Biophys. J. 2006, 90, L58-L60.
8 Zecher, K.; Aitha, V.P.; Heuer, K.; Ahlers, H.; Roland, K.; Fiedel, M.; Philipp, B. J. Microbiol. Methods 2018, 146, 10-114.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






