Hematite Pigments with Special Chemical and Physical Properties

Synthetic iron oxide red pigments with hematite modification are an important group of pigments that are produced industrially by different methods, including not only the Copperas, precipitation and Penniman processes, but also the Laux method and the Ningbo Process, developed recently by LANXESS and based on patented technology.
The Ningbo method supports sustainable process management in the production of red pigments and has already been implemented by LANXESS at its new production plant in Ningbo, China. A detailed description of the process was provided in the February 2015 issue of PCI.1
In this innovative process, a fine hematite seed and iron in the presence of ferrous nitrate are grown in a controlled process to form an unusually pure red pigment with high color saturation. The current process conditions achieve that only a small portion of the transition metals, such as manganese and chromium, are incorporated in the hematite lattice.
These new Ningbo Red pigments differ distinctly in terms of color from those manufactured by the Laux method. Compared with Bayferrox® 110, a Laux Red already established in the market, the yellow value b* for Ningo Red is up to 2.5 CIELab units higher, and the Cab* color saturation value is up to 3 CIELab units higher.
In this article, various methods are used to investigate why nitrate red pigments from the Ningbo Process display these special color characteristics and how the electronic structure has an influence on these color effects.
Chemical and Physical Properties
Important insight into this particle collective is provided by a scanning electron microscope (SEM): Figures 1a and 1b show a bright Ningbo Red and the Laux Red Bayferrox 110 at a magnification of 120,000.
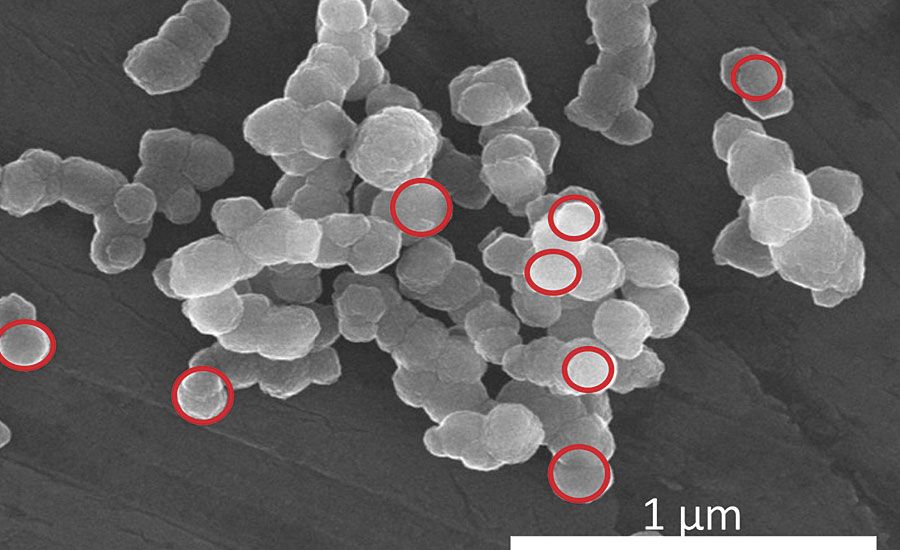
FIGURE 1a » Scanning electron micrograph of Ningbo Red.
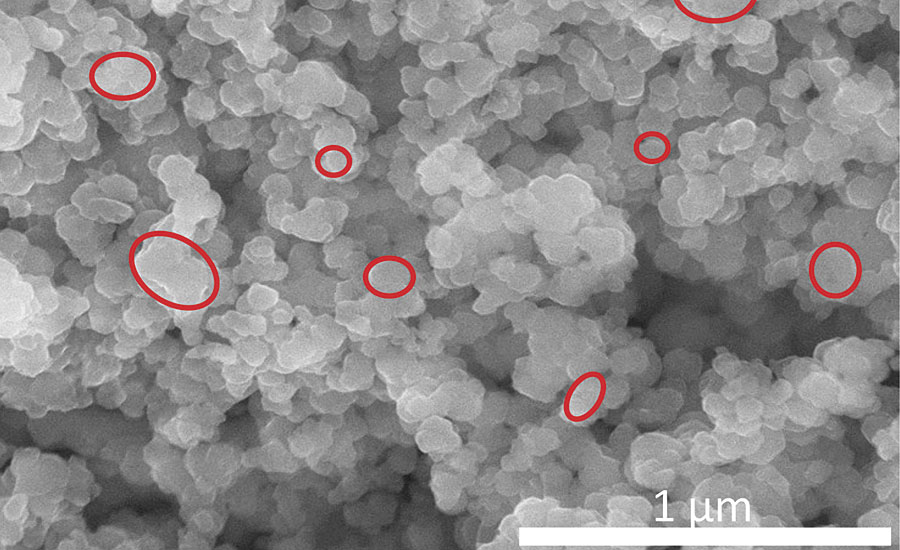
FIGURE 1b » Scanning electron micrograph of Laux Red 110.
In the case of the Ningbo Red, the primary particles have an isometric habit with a prevailing mean particle size of approximately 0.14 µm. The distributional width is relatively narrow in the range from 0.1 to 0.16 µm. The primary particles are joined in agglomerates or loose aggregates, and therefore can be distributed relatively easily in a binder.
The Laux Red Bayferrox 110 is a red pigment produced by means of calcination at 850 °C, and it displays primary particles with an irregular habit. The prevailing mean particle size is in the region of 0.20 µm, and the distributional width is relatively wide. Furthermore, pronounced aggregation resulting from the calcination process can be observed.
In the case of the bright Ningbo Red, the primary particles come very close to the ideal geometry of a sphere, where the mean particle size of 0.14 µm is significantly below the particle size of approx. 0.23 µm for maximum light scattering power, one consequence of this being that a brighter, yellow-cast color tone is achieved.
Other important properties of the two pigments are shown in Table 1. Major differences exist in terms of moisture and transition metal content, which can be attributed to process control. For the Ningbo Red pigments, processing takes place in the aqueous phase, meaning that water molecules are taken up in the pigment, while only small quantities of transition metals, such as manganese and chromium, are incorporated in the hematite lattice.
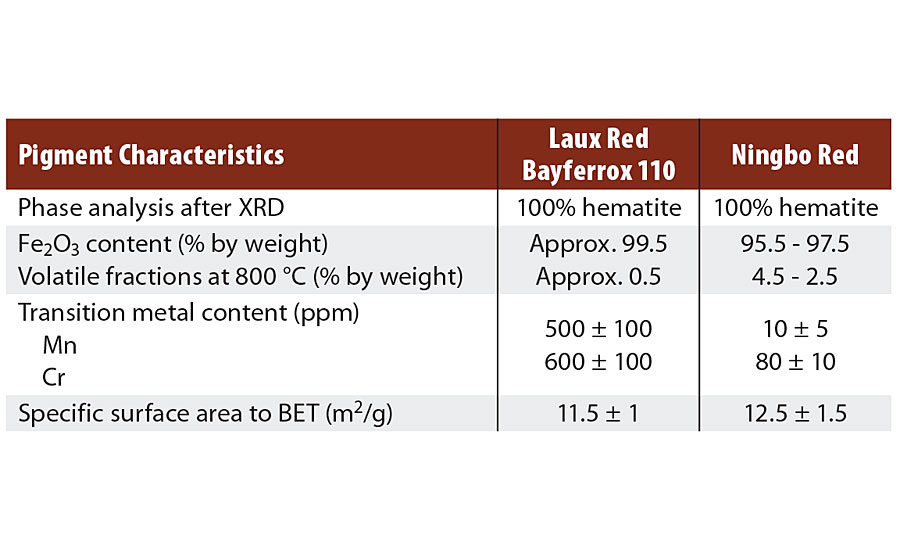
TABLE 1 » Characteristics of hematite red pigments.
The opposite is true of the LAUX Red Bayferrox 110, because calcination only permits moisture to be absorbed on the surface at a level of about 0.5% by weight. The transition metal content is high, this being caused by the iron raw materials. In both cases, XRD shows phase-pure hematite with comparable lattice constants.
Evaluating the UV VIS Spectra
Figures 2a and 2b show the absorption spectra for Ningbo Red and Bayferrox 110 in the wave number range from 5,000 to 25,000 cm-1, measured at room temperature. The absorption intensity is given on the y-axis as K/S and supports only qualitative conclusions for powdered pigments. The x-axis represents the energy scale (wave number n) and indicates high light transmission for both red pigments up to about 16,500 cm-1, which results in the red color. In the range from 16,000 to 17,000 cm-1, absorption rises very sharply with a wide plateau extending into the UV range. The two low-intensity bands I (approx. 11,500 cm-1) and II (approx. 15,500 cm-1) represent electronic transitions of the d5multielectron system of the Fe2O9 double octahedra in a high-spin state, where band II has considerable influence on color quality. This band prevents a sharp edge-like rise at the onset of absorption in the region of 16,500 cm-1, thus reducing the color intensity of the hematite red pigments.
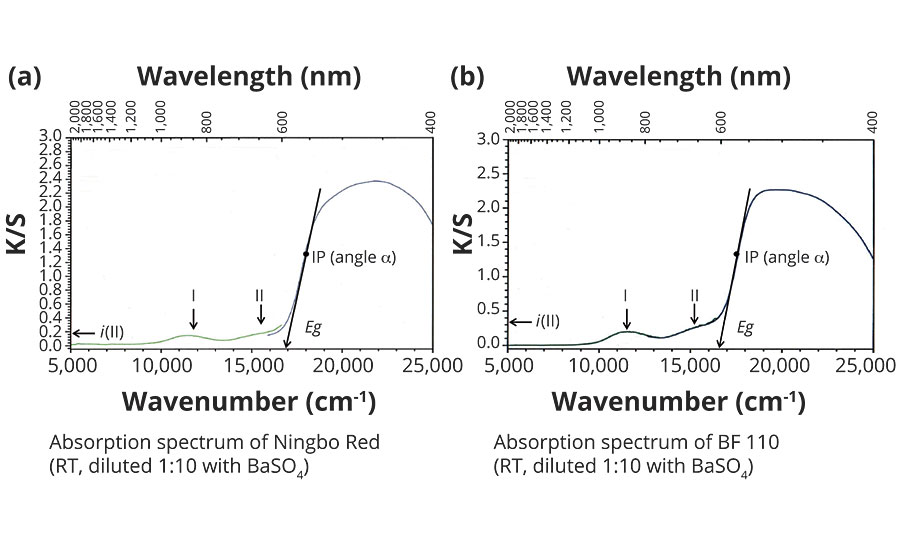
FIGURE 2 » Absorption spectra of hematite red pigments.
The high-intensity absorption increase between 16,500 and 19,000 cm-1 is caused by the band structure of the a-Fe2O3, with electronic transitions from the occupied valence band to the empty conduction band. Analyzing the absorption increase can help to determine characteristic spectroscopic values, which are well-suited to explaining the color differences.
First, the respective inflection point IP is determined graphically and, at this point, the angle of inclination a estimated via the tangent. The tangent at inflection point IP, when projected onto the x-axis (energy scale), indicates in a first approximation the energy gap Eg of the red pigments, allowing a conclusion to be made about the yellowish cast of the red pigments.
The intensity of band II is determined at 16.000 cm-1 by projection onto the y-axis as i(II) and provides dimensionless approximations.
The steepness of the absorption increase is comparable for both red pigments, because an angle a of approx. 80° was determined for each. This result can be explained by the virtually identical band structures.
In contrast, a significant difference can be observed in the intensities i of band II, i.e. approx. 0.33 for Bayferrox 110 and 0.15 for the Ningbo Red, this being attributable to the very high transition metal contents and coarser primary particles of the Laux Red. The transition metals, particularly Mn3+ and Cr3+, occupy octahedral sites in the hematite lattice, meaning that other electronic transitions in the region of 15,500 ± 1,000 cm-1 are possible and additionally increase the absorption intensity i(II).2
The two energy gaps Eg differ by approx. 350 cm-1, i.e. the Ningbo Red has a somewhat larger energy gap and thus a higher yellow value (b*). An explanation of this effect is mainly provided by the smaller primary particles in the Ningbo Red, because the band can bend open slightly in the region of the surface.2
Water Molecules in the Hematite Lattice
Ningbo Red pigments display a loss in mass of 2.5 to 4.5% by weight in thermogravimetric analysis (TGA) up to 800 °C. This loss in mass occurs in steps and, on account of the reaction conditions, can only be attributable to moisture in the form of water.
A suitable method for detecting H2O molecules in the hematite is IR spectroscopy, which displays the typical vibrational bands of the H2O in the energy range from 800 to 4,000 cm-1. Figure 3 shows the IR spectrum of a nitrate red pigment with approx. 3.5% by weight moisture, measured at room temperature. The very high-intensity Fe-O lattice vibrations of the Fe2O9 double octahedra are in the region of 350 to 650 cm-1.
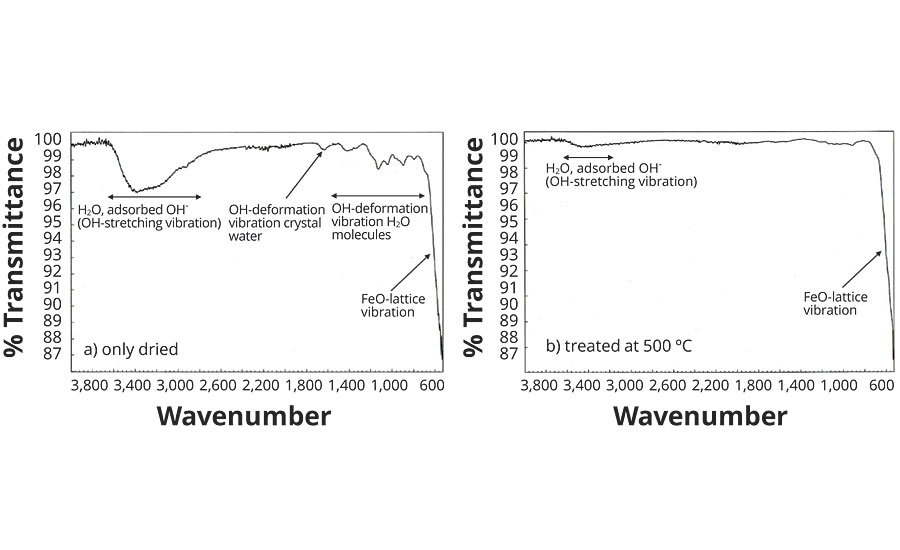
FIGURE 3 » Infrared spectra of Ningbo Red Pigments.
A number of low-intensity bands can be seen between 800 and 1,650 cm-1, which are attributable to the O-H deformation vibrations of the H2O molecule (Table 3). A very wide band of moderate intensity is registered in the range from 2,800 to 3,600 cm-1, this being caused by different O-H valence vibrations. The widening of the band (approx. 800 cm-1) results from H bridge formation between the H2O molecules and the O2- ligands in the hematite lattice.
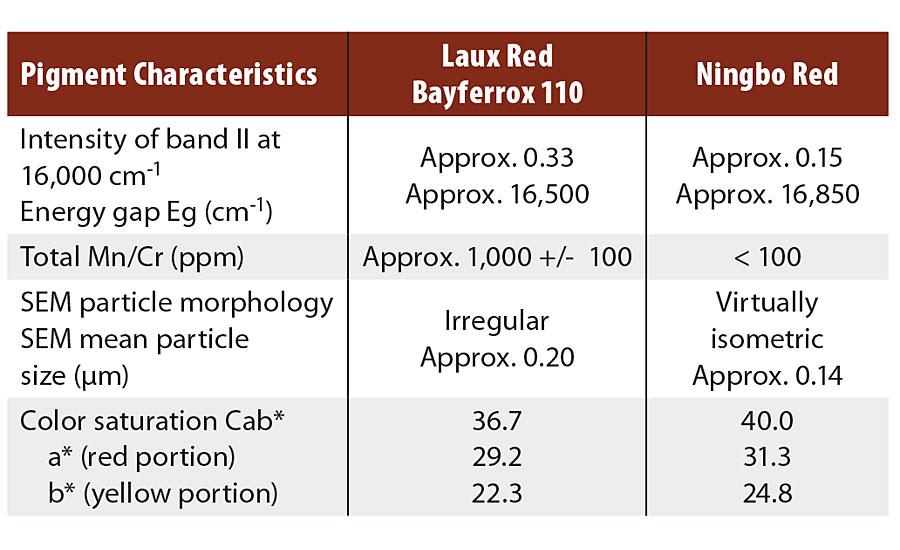
TABLE 2 » Pigment characteristics and color values of hematite red pigments.
Looking at the IR spectrum of the same nitrate red pigment after temperature treatment at 500 °C (Figure 3), all bands between 800 and 1,650 cm-1 have disappeared, and the intensity of the wide bands at approx. 3,300 cm-1 has decreased considerably. The residual moisture has decreased to around 0.8% by weight (Table 3) and is present primarily as adsorbed water on the pigment surface.
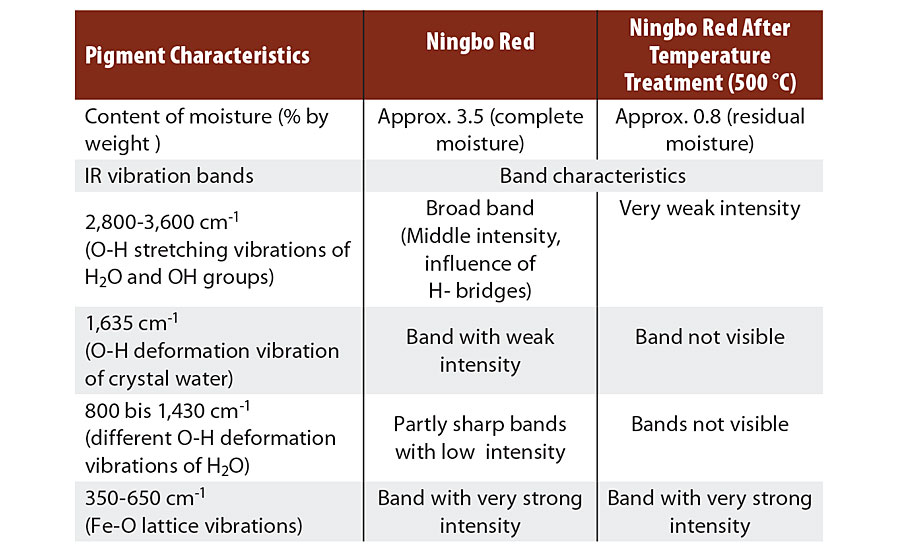
TABLE 3 » Analysis of the IR spectra of Ningbo Red pigments.
It is interesting to consider whether the water of crystallization has any effect on color. The incorporation of H2O molecules in the octahedral gaps possibly results in a general increase in the ionic character of the Fe-O bonds, which could lead to slight enlargement of the energy gap Eg and thus support the formation of bright, yellow-cast red pigments.
Conclusion of the Investigations and Outlook
Bright, yellow-cast hematite red pigments made by the LANXESS Ningbo Process possess unique color properties primarily attributable to a very low transition metals content and isometric particles with a narrow distribution width. Compared with the LAUX Red Bayferrox 110, the yellow value b* is up to 2.5 CIELab units higher and color saturation Cab* up to 3.0 CIELab units higher.
Analysis of the absorption spectra provides spectroscopic characteristic values, such as energy gap Eg, which depends mainly on particle size and intensity i of the absorption bands II, which depend on particle size and additionally on the manganese/chromium content. The water incorporated in the hematite lattice in the form of H2O molecules has only a slight influence on the color characteristics.
After an intensive testing phase, the LANXESS Inorganic Pigments business unit has successfully launched different types of yellow-cast hematite pigments onto the market, selling them in its Bayferrox 500 product line. LANXESS continues to invest in the development of innovative red pigments, its goal being to steadily expand the color space while at the same time achieving improved pigment characteristics. The color potential of iron oxide red pigments is far from exhausted. For example, fine-tuning the parameters of the manufacturing process can positively influence the chromaticity of the pigments, which is affected by factors such as particle size and shape, porosity and particle size distribution. Based on existing experimental results and theoretical calculations, it is evident that the current color limits of iron oxide red pigments can be expanded in the near future.
References
1 Czaplik,W. , Paint & Coatings Industry magazine, February 2015.
2 Article of LANXESS to be published.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








