How the Design of (Poly)isocyanate Hardeners Can Impact the Properties of Aliphatic Polyurea Coatings

One hundred-percent-solids or very low-VOC aliphatic polyurea coating systems are of high interest due their high reactivity, enabling high application productivity, the ability to potentially generate high film build as well as an extended application window at low temperature and high humidity conditions. However, these systems may require, depending on the application, improvements in terms of application viscosity (for 100%-solids systems) and pot life. This article shows how these targets can be achieved by using the appropriate polyisocyanate structure. It will more particularly highlight the interest of a specific difunctional isocyanate polymer, and how the overall performance of protective coatings can be improved with this material.
Introduction
Polyaspartic acid ester type resins were introduced at the end of the 1980s as reactive diluents to increase the solids content of the polyol part of two-pack high-solids polyurethane coatings.1 In the last two decades, these systems have gained an increasing interest in different fields of application due to their distinctive properties. In combination with aliphatic polyisocyanates, they enable the design of fast-drying coating formulations with moderately high film build (up to about 300 µm DFT) and low to zero VOC content. Their most prominent uses are found in flooring and protective applications, where their high film build and cure speed lead to higher productivity (lesser coating layers) and faster return to service.2-3
The interest in these systems is such, that today several resin manufacturers supply polyaspartic acid ester type resins, and more recently alternative systems like polycarbamide resins4 were introduced into the market of this class of polyurea coating technology.
Typical aliphatic polyurea systems are very fast, 100%-solids systems based on primary and secondary amines with gel times of some seconds due to their very high reaction rates with the isocyanate functions of the prepolymers. Two-volume spray systems are required to apply these systems, making the application of several-millimeter-thick dry films possible.
Compared to the hydroxyl/isocyanate reaction, the amine/isocyanate reaction is very fast, does not require any catalyst for accelerated curing and cures still at very low temperatures. Due to the steric hindrance of the employed secondary amine groups, polyaspartic acid ester resins exhibit much lower reactivity with isocyanate groups than the more conventional polyurea systems. The degree of hindrance of the secondary amine groups imparted in the resin structure controls the reactivity with the isocyanate groups of the reaction partner, thus resins with different reactivity can be designed. However, this class of coating system cures significantly faster than traditional catalyzed aliphatic two-pack polyurethane systems. Environmental humidity acts as a catalyst for the reaction of polyaspartic acid esters with isocyanates and can accelerate curing after application.
The low viscosities of the employed secondary hindered amines allow the formulation of coating systems with lower or no VOC. Depending on the formulation, these systems can be applied by traditional application techniques like spray, roller or brush. These systems are generally crosslinked with conventional aliphatic HDI- or IPDI-based polyisocyanates.
The application and final properties of the coating formulations can be adjusted, a) by the choice of the hindered amine resin(s), and b) by the polyisocyanate structure used for the crosslinking. The adjustment of the amine resin systems governs essentially the reactivity. In this study, we have used two different polyaspartic ester resins with different reactivity towards isocyanates. The properties of the films obtained have been studied using different aliphatic (poly)isocyanate structures, as described below.
Experimental
Raw Materials and Preparations
The raw materials used in this study (polyisocyanates and polyaspartic resins) are described in Table 1 and Table 2 respectively. Tolonate™ X FLO 100 is a partially biobased, very low-viscous, aliphatic HDI-based polymer. In our study, it was used as a “reactive diluent” in the other conventional biuret or trimer polyisocyanates.
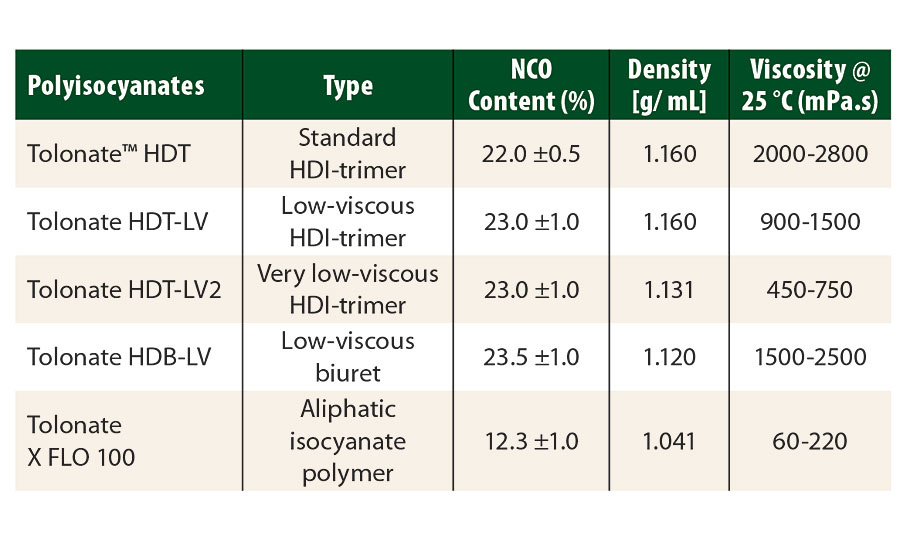
TABLE 1 » Aliphatic HDI-based polyisocyanates, supplied by Vencorex.
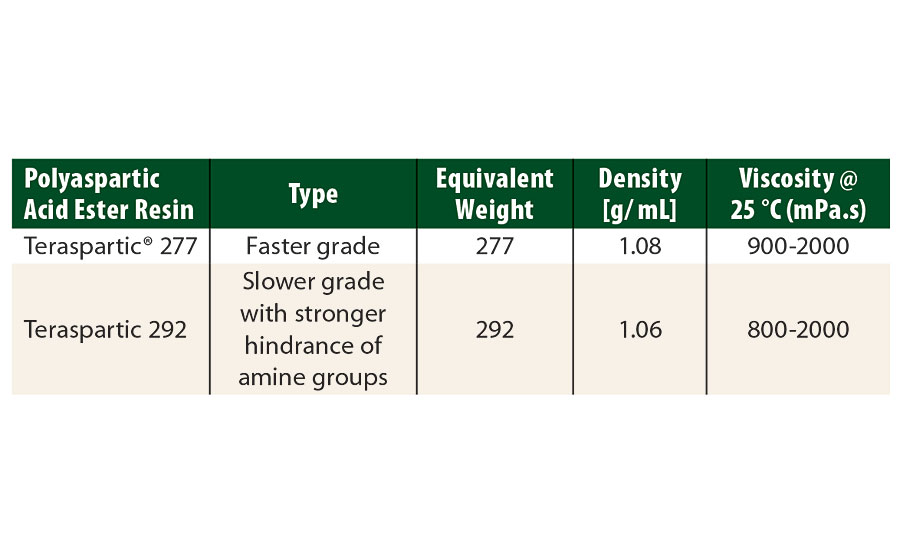
TABLE 2 » Polyaspartic acid ester resins, supplied by Pflaumer Brothers Inc.
Using these resins, model systems with a NCO/NH=1.0 stoichiometry were formulated to study rheological properties. The protective white topcoat formulation (Part A) used in this study is described in Table 3. Tolonate HDT-LV2 and Tolonate X FLO 100 were used as crosslinkers (Part B). The solids content of the isocyanate part was adjusted at 70% solids content in butyl acetate. The protective topcoat was spayed at about 60 µm DFT over pre-primed steel panels to a total DFT of about 100 µm.
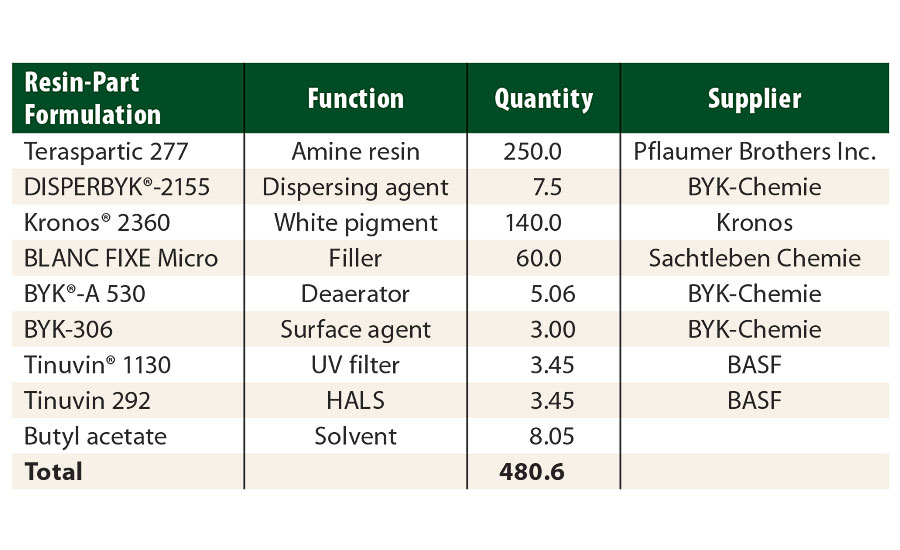
TABLE 3 » Resin-part formulation of the study’s white protective topcoat.
Rheological Evaluation of Viscosity Evolution and Buildup of Mechanical Properties
The rheological experiments were conducted using a MCR 302 rheometer supplied by Anton Paar, with a 25 mm plate/plate geometry. Measurements were obtained at 25 °C and 40 °C. The temperature was controlled by a Peltier plate and an upper Peltier stove, assuring homogeneous temperature distribution. Environmental atmosphere was controlled by a continuous flow of dry air. The measurements were started right after admixing the A and B components and their charging into the measurement device – the delay for mixing and charging being about 2-3 minutes. The crosslinking reaction of the system and the buildup of the polymer network were monitored using small-amplitude dynamic oscillatory time sweeps. The multiwave experiments were conducted at a base frequency of 0.1 rad s-1 and its harmonics (2, 4, 7, 10, 20, 40, 70, 100, 200 and 400) with measurement points every 100 s. A constant strain amplitude of 1% was applied to ascertain a deformation within the linear viscoelastic region (LVR).
Evaluation of Reaction Kinetics
The consumption of isocyanate groups (NCO) over time by reacting with amine functions was calculated from the evolution of the NCO peak in the Fourier transform infrared spectra observed with a Bruker Tensor 27 FTIR spectrometer used in ATR mode.
Evaluation of Pot Life
The pot life of the systems was determined by flow time measurements with a DIN n°4 flow cup by measuring the time needed for doubling the flow time following ISO 9514.
Evaluation of Drying Times
The drying times were measured with an Erichsen dry-time recorder Coatmaster 509 MC for 150 µm applied wet films.
Evaluation of Film Adhesion
The adhesion of the topcoat was evaluated by crosshatch tests according to ISO 2409 standard.
Evaluation of Tensions within the Cured Films
The internal tensions inside the films were evaluated for films applied with a wire rod at 150 µm wet onto 2A type LENETA cards (254 x 140 mm) on a length of 220 mm (black and white area). The tension was evaluated by the bending/curvature of the cards upon drying and storage of the films under controlled standard conditions at 23 °C and 50% r.h. The radius of curvature R is measured directly or is derived from the end-to-end distance d of coated length L (Figure 1), admitting a constant curvature of the coated section.
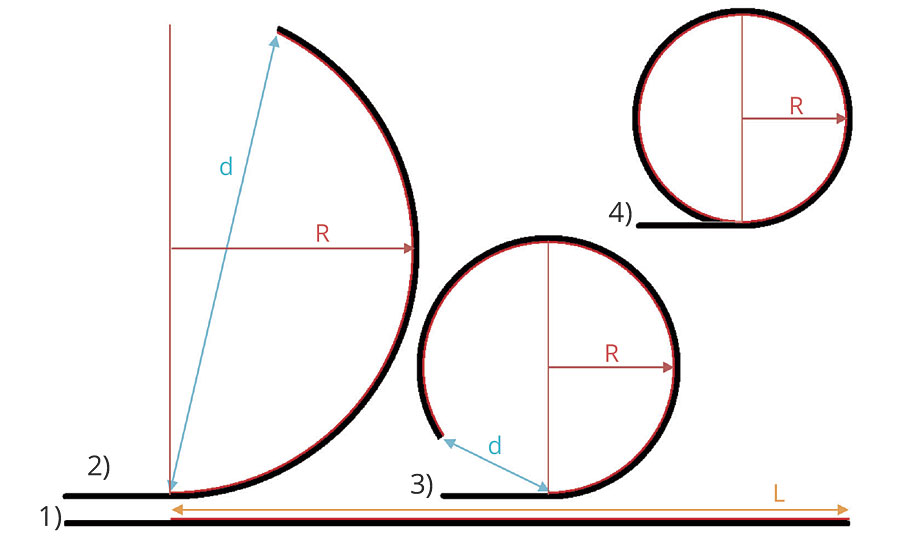
FIGURE 1 » Scheme of the internal coating tensions causing curvature of 2A type LENETA cards: 1) freshly coated straight card, with coating length L; 2) and 3) by the internal film tension/shrinkage curved card for which the radius of curvature R is derived from the end-to-end distance d of the coated length L; 4) fully enrolled card for which the radius of curvature R can be measured directly.
Evaluation of Film Flexibility
The flexibility of the films was evaluated by measuring their resistance to cracking upon conical mandrel bend tests following ASTM standard D522.
Evaluation of Impact Resistance
The reverse impact resistance was evaluated following ISO 6272 standard.
Evaluation of Chemical Resistance
The chemical resistance was evaluated by covered spot tests following standard ASTM D 1308 for the substances listed in Table 4. The evaluation scale for the chemical spot test reaches from 0 (for an unaltered film) to 5 (for a destroyed film).
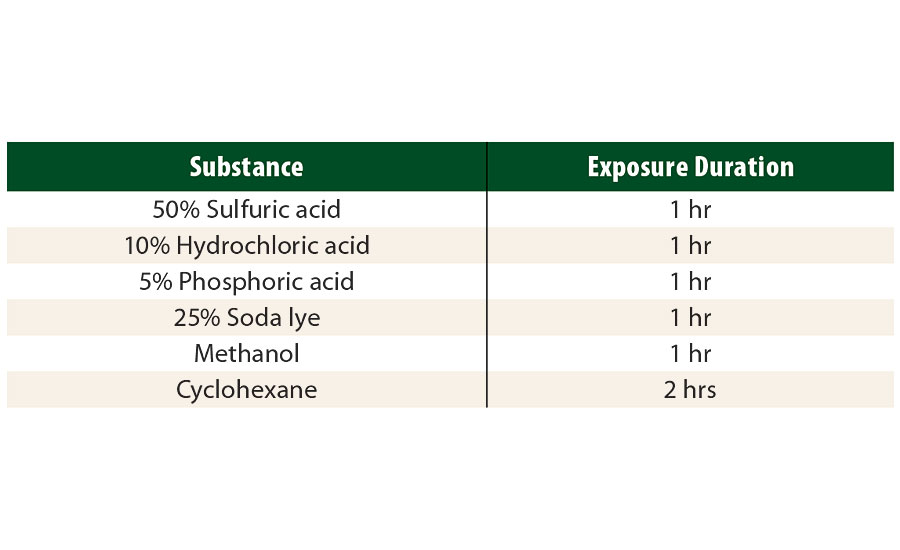
TABLE 4 » Chemical substances and respective exposure times for which the resistances have been tested.
Evaluation of UV Stability
The coating systems were tested in an UVB-313 test cycle following the accelerated UV light-stability test standard ASTM G53.
Evaluation of Corrosion Resistance
The corrosion resistance of the coating systems was evaluated in a 28-day salt spray test cycle following ASTM B 117.
Contact Angle Measurements
The contact angles with demineralized water were measured for films of polyaspartic ester resin-based crosslinked systems in order to determine the surface tension of the polymer film.
Results and Discussion
Impact of the Reactive Diluent on Viscosity and Crosslinking Kinetics
Tolonate X FLO 100 has a significantly lower viscosity than usual HDI-based polyisocyanates, with which it is blended. Hence the resulting blend viscosity decreases with an increasing amount of reactive diluent.
More interesting is the impact of the polyisocyanate composition on the viscosity of the mix between the resin part A and the polyisocyanate part B (Figure 2), and the reaction kinetics of the system A+B (Figure 3).
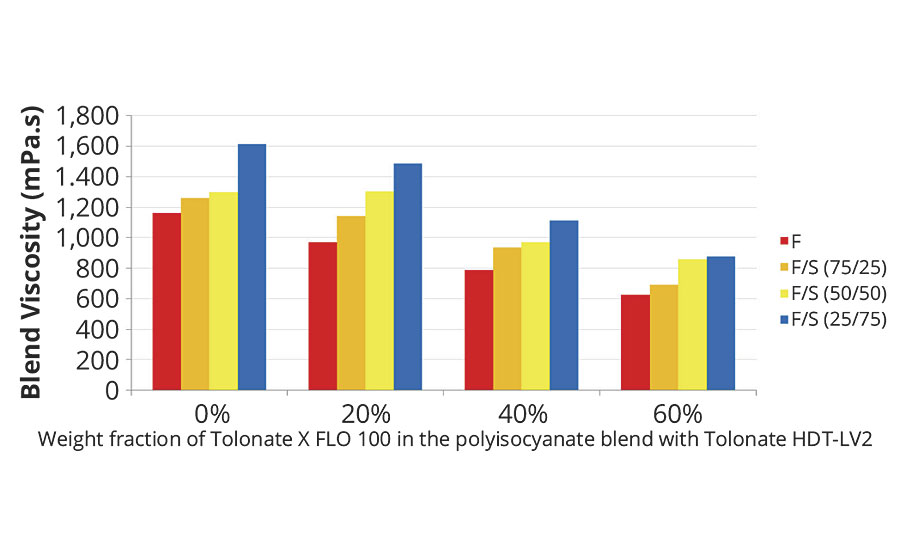
FIGURE 2 » Initial blend viscosities for blends of fast (F) and slow (S) polyaspartic acid ester resins at different blend ratios, crosslinking at a stoiciometry of NCO/NH=1.0 with polyisocyanate blends composed of Tolonate HDT-LV2 and X FLO 100 at different weight fractions of the reactive diluent.

FIGURE 3 » NCO conversion for blends of the fast polyaspartic ester resin (F) with blends of low-viscous HDI-based polyisocyanate with the reactive diluent at stoichiometry NCO/NH=1.
Right after admixing of A+B, the viscosity of the reacting system rapidly increases, exhibiting an exponential development with time, as seen on Figure 4. Consequently the initial viscosity of the blend cannot be properly measured. By using a regression of an exponential model, we can however determine the viscosity at t=0, taking into account a lapse of 2 minutes required for charging the admixed sample and starting the measurement procedure. The results are displayed in Figure 2 for several polyaspartic resins and polyisocyanate blends. The viscosity reduction of the initial blend viscosity is quite significant already at 20 or 40% weight fraction of the reactive diluent, depending on the targeted application.
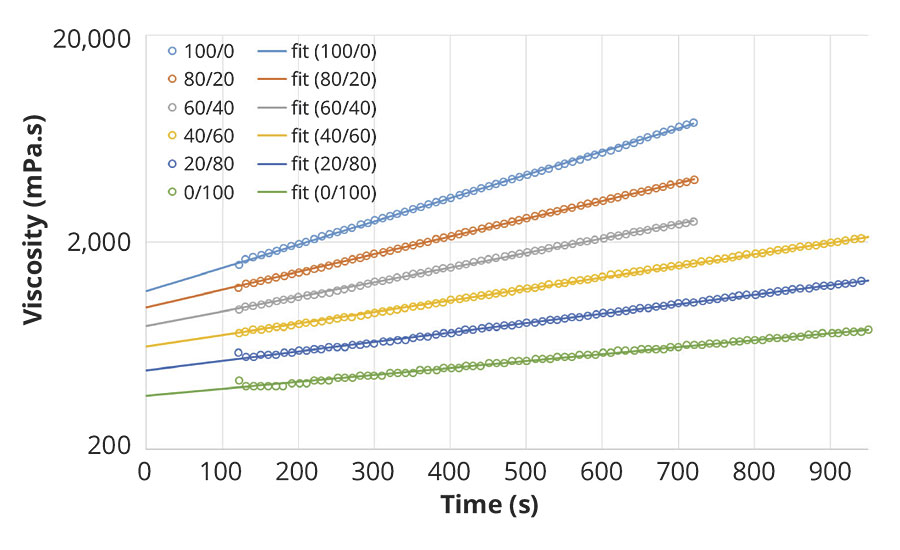
FIGURE 4 » Early viscosity evolution upon crosslinking of a polyaspartic ester resin, Teraspartic 277, with blends of a low-viscous HDI-based polyisocyanate, Tolonate HDT-LV2 and the reactive diluent, Tolonate X FLO 100, at stoichiometry NCO/NH=1. The polyisocyanate blends are indicated with their respective weight ratio between low-viscous HDI-based polyisocyanate and the reactive diluent.
The NCO conversion measured for different HDI-based polyisocyanate blends reacting with a given polyaspartic ester resin, mixed at fixed stoichiometry (NCO/NH=1) is fairly independent of polyisocyanate composition.
However, at a given stoichiometry, the reactive diluent has an impact on:
- the density of isocyanate functions per volume unit of the system (Figure 5a), since equivalent weight and density of the blended polyisocyanates vary with the blend ratio of the HDI-based polyisocyanate and the reactive diluent;
- the average functionality of the polyisocyanate blend, since the fraction of reactive diluent with its functionality close to 2 contributes essentially to chain extension and does not add branch units (functionality ≥ 3) (Figure 5b).
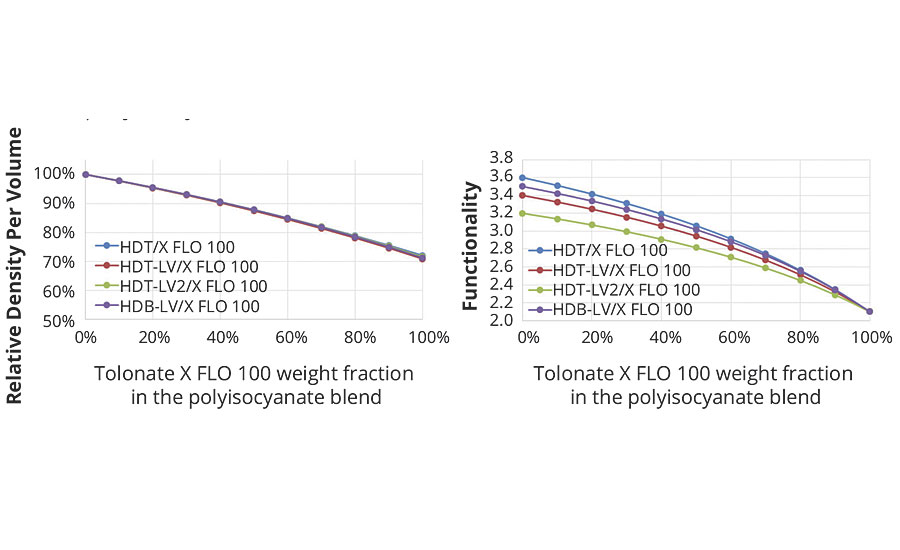
FIGURE 5 » The left graph (5a) shows the decrease of the density of crosslinking functions per volume for blends of a low-viscous HDI trimer with the reactive diluent at a given stoichiometry (NCO/NH = 1); the right graph (5b) shows the evolution of the average functionality of the polyisocyanate blend.
These two effects of the blending of polyisocyanate trimers with the reactive diluent have an impact on the polymer network built-up over time. As shown, the chemical reactivity between isocyanate groups and secondary amine groups can be considered as not being altered by the blend ratio of the HDI-based polyisocyanate with the reactive diluent. However, the density of isocyanate functions per volume and the average polyisocyanate functionality decrease, and will impact the branching of the growing network and the final crosslinking density. Consequently, the viscosity development prior to reaching the gel point of the system and therefore the pot life will be affected.
Gelification of a crosslinking system occurs only if the reacting system is capable to build an infinite network. Flory and Stockmayer5-7 have deduced from statistical considerations a branching coefficient a describing the probability that a given functional group of a branch unit leads via a chain of bifunctional units to another branch unit; branch units being functional groups with a functionality of 3 or more. Assuming that all functional groups of two reacting molecule groups, A and B, have equal reactivity, and that the B-functional units are bifunctional, the branching coefficient can be written as:
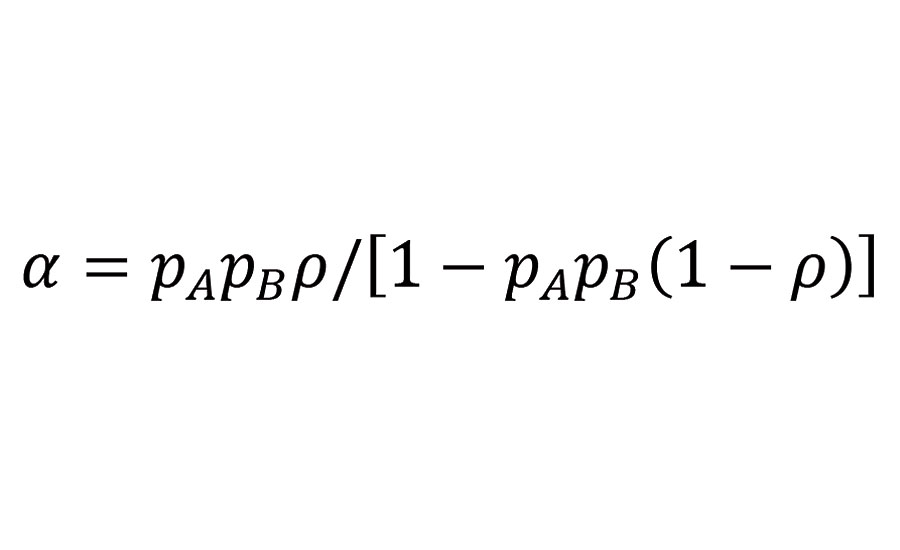
Equation 1
Where pi is the probability that the functional group i has reacted, and r is the ratio of functional groups A (reacted and unreacted) belonging to branch units to the total number of A groups in the mixture. With r being the ratio of A to B groups initially present in the mixture, the probabilities pi are related as follows: pB=rpA.
In the present case where the mixture of the reactive groups is at stoichiometry (r=1) and the functional groups A and B correspond respectively to secondary amine groups and isocyanate groups, the branching coefficient becomes:
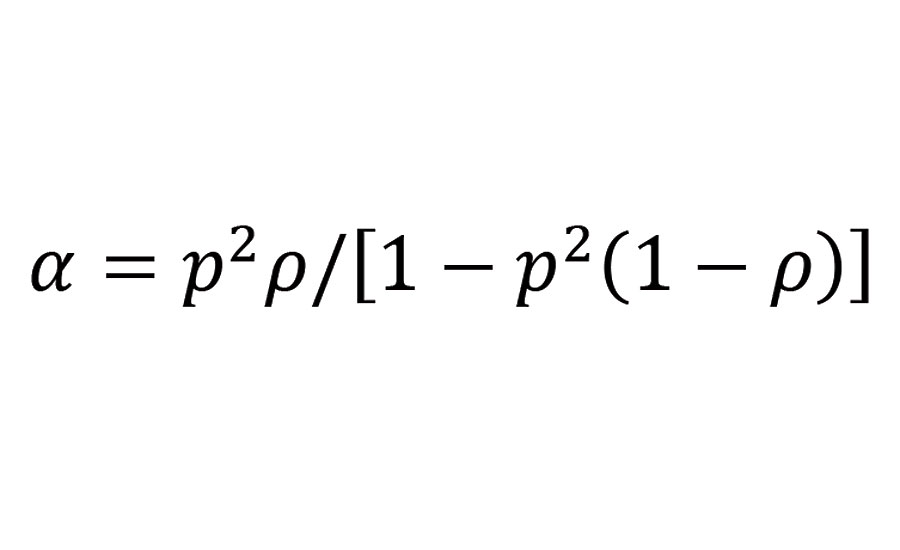
Equation 2
The critical value of a at which the formation of an infinite network (gel) becomes possible has been calculated by Flory and Stockmayer as follows:
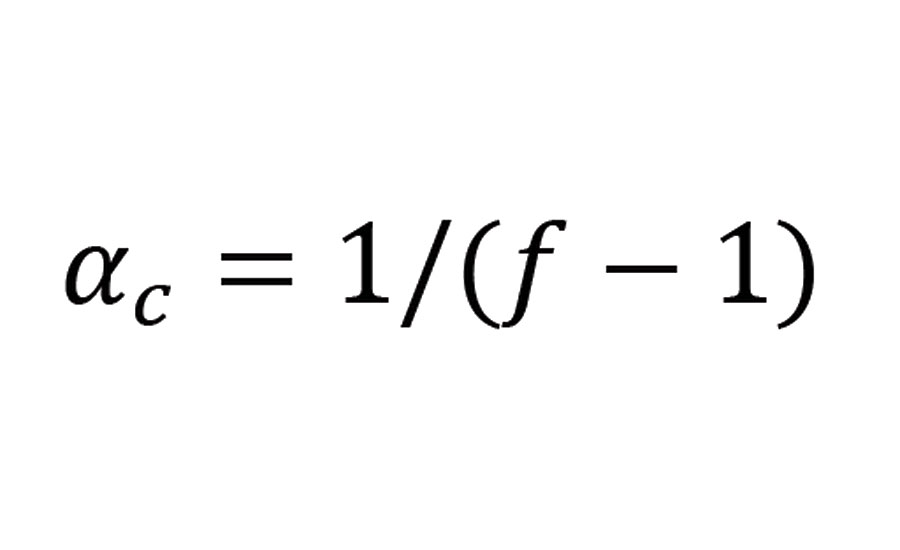
Equation 3
where f is the functionality of the branching groups.
This enables to calculate the critical branching coefficient for our present system as a function of the average functionality of the blends of different HDI trimers with the reactive diluent, as shown in Figure 6.
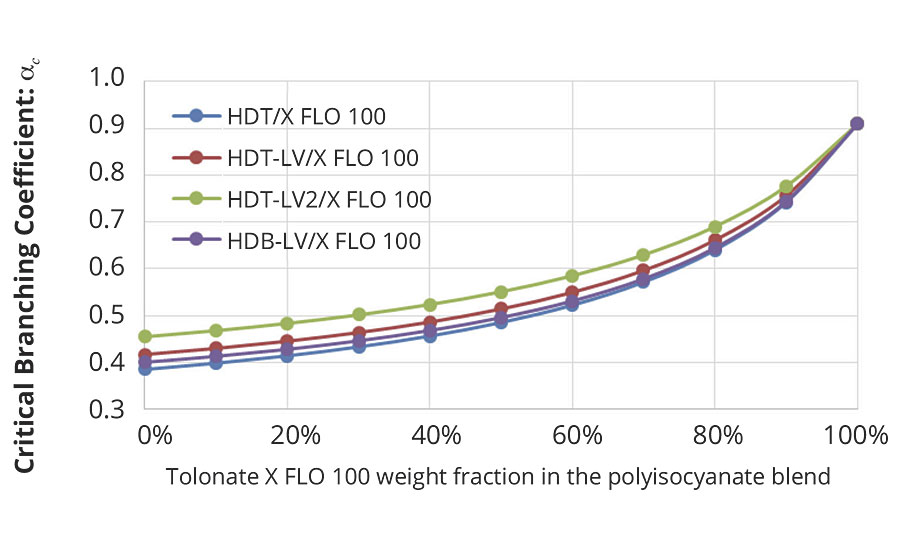
FIGURE 6 » Critical branching coefficient calculated for the mix of polyaspartic ester resins at stoichiometry (NCO/NH=1) as a function of the polyisocyanate blend.
Also, from equations 2 and 3, the critical conversion to form a gel can be calculated as a function of the ratio r and the average functionality of the polyisocyanate blend:

Equation 4
In our case, the critical conversion necessary to form a gel shown in Figure 7 is only dependent of the average functionality of the polyisocyanate blend and the ratio between the number of NCO groups being part of branch units to the total number of NCO groups, r.
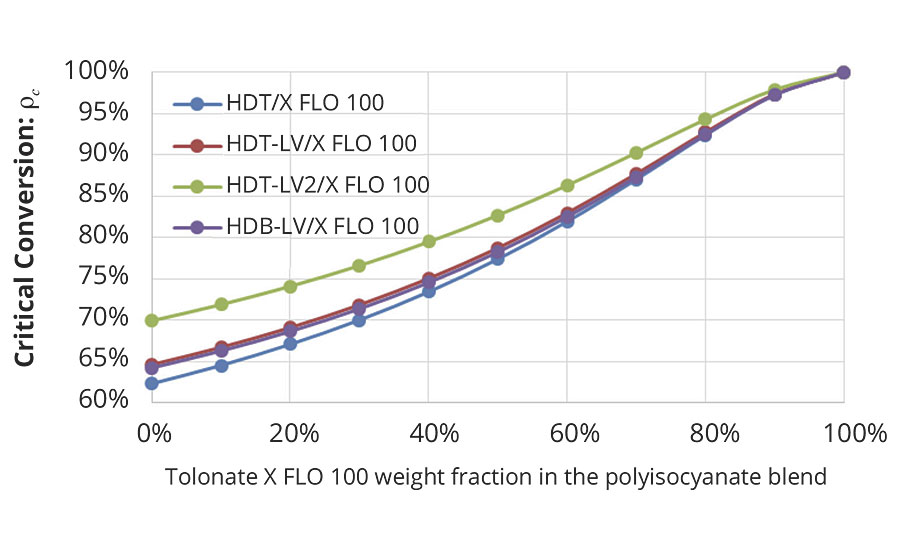
FIGURE 7 » Critical conversion to form a gel calculated from the average functionality of the different polyisocyanate blends with the reactive diluent and the ratio (ratio of NCO groups (reacted and unreacted) belonging to branch units to the total number of NCO groups in the mixture).
Based on the measured time dependence of the conversion p (Figure 3) it is possible to estimate the time necessary to reach the critical conversion pc required for a given system to form a gel (Figure 8).
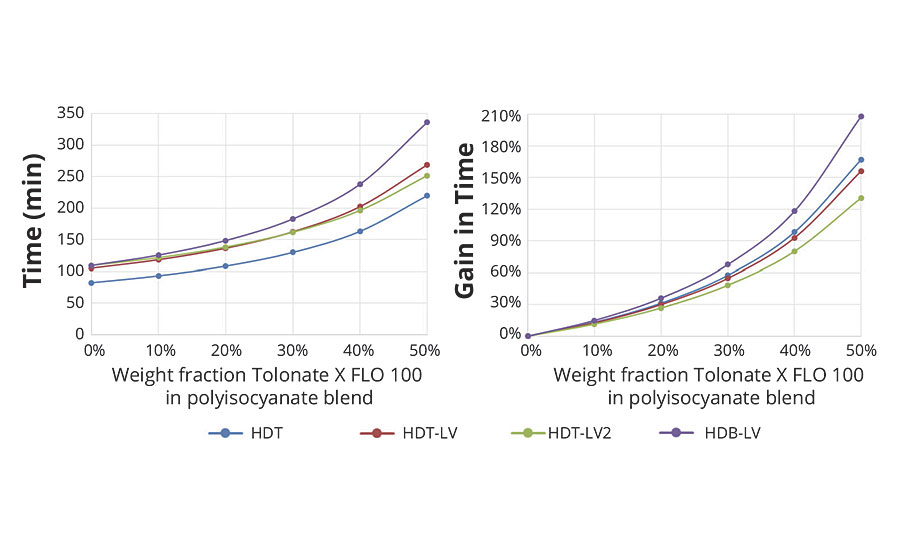
FIGURE 8 » Estimation of the time required to form a gel for the various systems as a function of the polyisocyanate composition.
Hence, the potential impact of the reactive diluent in a polyisocyanate blend on the gel time of the system can be estimated, as shown in Figure 9.

FIGURE 9 » Gain of time to form a gel as a function of the weight fraction of the reactive diluent with different HDI polyisocyanates types.
As shown earlier, the viscosity development with time can be approximated in the early stages of the crosslinking process by an exponential equation, allowing formulators to determine a time constant and thereby assess the necessary time to double viscosity.
The gain in time to double viscosity coming from the reactive diluent contained in the polyisocyanate blend is shown in Figure 10. The results demonstrate that the presence of reactive diluent in the hardener systems at rather low concentration (20%) will have a significant impact on the pot life of the system during the application process, whatever the resin composition used is.
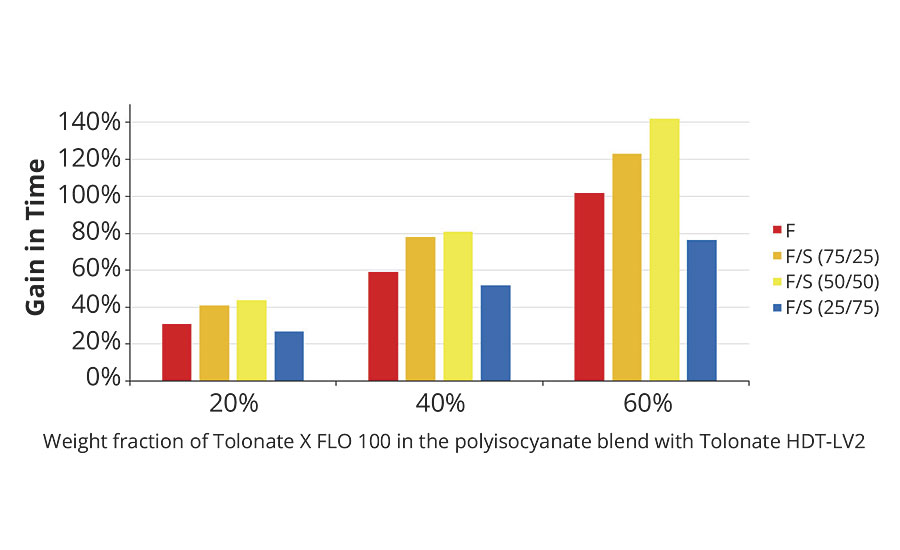
FIGURE 10 » Gain in time for doubling the viscosity of the polyaspartic ester system as a function of the reactive diluent content.
The evolution of the network build up and the gel formation over time has been monitored by Dynamic rheology measurements. When the value for the elastic modulus (G') becomes more important than the viscous modulus (G"), the system can be considered to be close to its gel point. However, the exact gel point of the system is obtained when the phase angle between the modules becomes independent of the frequency.8 In the present case it was not possible to determine the exact gel point, but we could approximate it by the cross-over point of G' (t) and G" (t).
The gel point has been determined approximatively for two systems based on the fast polyaspartic ester resin and HDI-based polyisocyanate blends with and without the reactive diluent. The results (Figure 11) are expressed as gain in time to reach the gel point for the polyisocyanate blends containing 20 wt% of reactive diluent, compared to blends without it. The measurements are compared to the gel times calculated with the help of the Flory’s and Stockmayer’s model.

FIGURE 11 » Gain in time to reach the gel point of polyaspartic ester systems formulated with two different polyisocyanates by using 20% by weight of reactive diluent in the hardener blend.
The measured and calculated values for the influence of the reactive diluent on the time required to reach the gel point match fairly well. And also the magnitude of gain in time to double the initial blend viscosity, though this phenomenon appears much earlier upon crosslinking of the systems (Figure 10), is comparable with these results.
The impact of Tolonate X FLO 100 in these systems on the time needed to double the viscosity or to reach the gel point of these systems clearly indicates its potential to increase the pot life of polyaspartic acid ester-based coating systems.
Impact on the Application and End Properties of a Protective Coating System
The impact of the reactive diluent on the viscosity increase and the time required for gelification observed for the formulation was evaluated for real life applications with a white protective topcoat. This gave further opportunities to evaluate the impact of the reactive diluent on the end properties and performances of the coating film.
As seen in Table 5, the results show for the system using NCO/NH ratio of 1.0 with a blend of Tolonate HDT-LV2/X FLO 100 (80/20) a pot life of about 18.5 min against 11.4 min for Tolonate HDT-LV2 alone. This presents a very significant advantage for the application and confirms the findings observed for the model system.
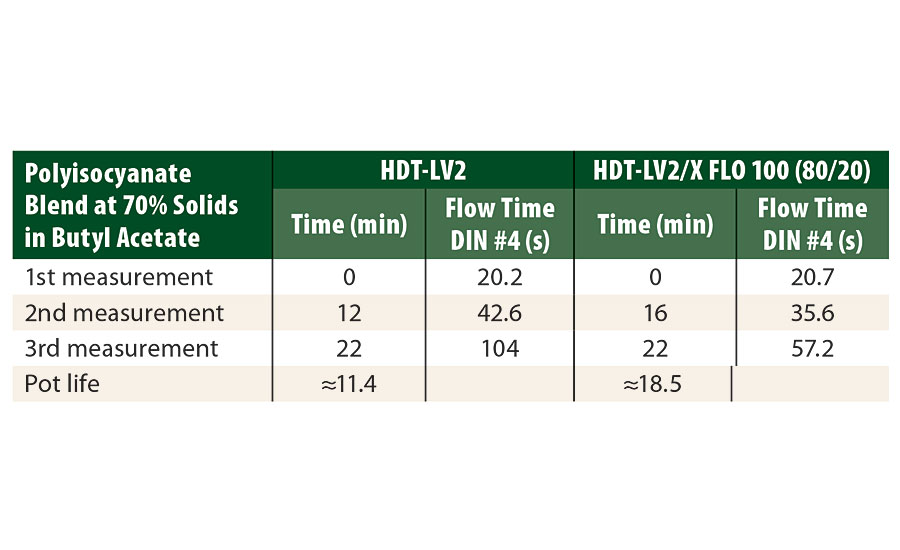
TABLE 5 » Pot life determination with DIN #4 cup flow time measurements for the white topcoat formulation crosslinked at a stoichiometric ratio of NCO/NH=1, with either Tolonate HDT-LV2 or a blend of Tolonate HDT-LV2/X FLO 100 (80/20), both respectively at 70% I butyl acetate.
The through-dry times of the applied wet films are similar: 31 min. without, against 33 min. with reactive diluent used in the crosslinker blend.
The results obtained for the film adhesion show an improvement from rating 2 without, to rating 0, perfect adhesion, with reactive diluent. These results are undoubtedly linked to the reduction of shrinkage and internal tensions, as shown in Table 6.

TABLE 6 » Evolution of border-to-border distance and radius of curvature of LENETA cards to which the protective topcoat has been applied, crosslinked with different polyisocyanate blends, after storage in controlled environmental conditions (23 °C, 50% r.h.).
Fast-reacting polyurea coatings tend to build up strong internal tensions upon crosslinking.9 As seen in Table 6, the curvature observed for the cards with films crosslinked without reactive diluent was significantly stronger than with reactive diluent.
The results show clearly reduced internal tension for the film crosslinked by a polyisocyanate blend containing the reactive diluent.
The film flexibility or cracking resistance has been evaluated with the conical mandrel bend test. No difference could be observed for the present formulation between films crosslinked with and without reactive diluent, none of them did show any cracking at the narrowest curvature of the test panels. Also, the reverse impact test did not show any significant weakening in the resistance of the film containing the reactive diluent. The coating containing the reactive diluent resisted to a fall height of 95 cm against 100 cm for the coating without reactive diluent, while both formulations passed the direct impact test.
The results of the chemical resistance spot tests (Table 7) show generally good performances for most of the tested substances, with slightly better results for the system containing the reactive diluent. However, we observed a weakness for methanol, which may be attributed to a slight decrease of the crosslinking density of the polymer network when using the reactive diluent in the hardener. Actually, the average functionality of the polyisocyanate blend containing the reactive diluent with a functionality of about 2 slightly decreases to 3.07 as compared to the “pure” trimer type (F=3.2).
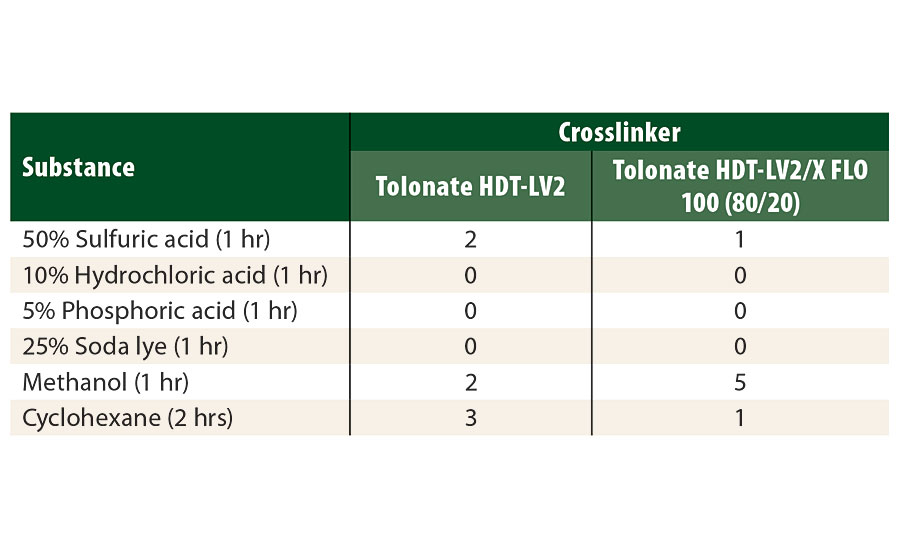
TABLE 7 » Chemical resistance evaluated by droplet tests for different substances (Scale: 0 (unaltered film) to 5 (film destroyed).
The coatings systems are found to be very stable in accelerated UV light-stability tests with over 2000 hrs exposure to a UVB-313 test cycle, exhibiting a color change of about ∆E*=1.0, independently from the hardener composition.
The corrosion resistance of the coating systems has been evaluated in a 28-day salt spray test cycle. The results show for the coatings crosslinked with a polyisocyanate blend containing Tolonate X FLO 100 a significant improvement of the corrosion protection compared to the reference coatings (Figure 12).
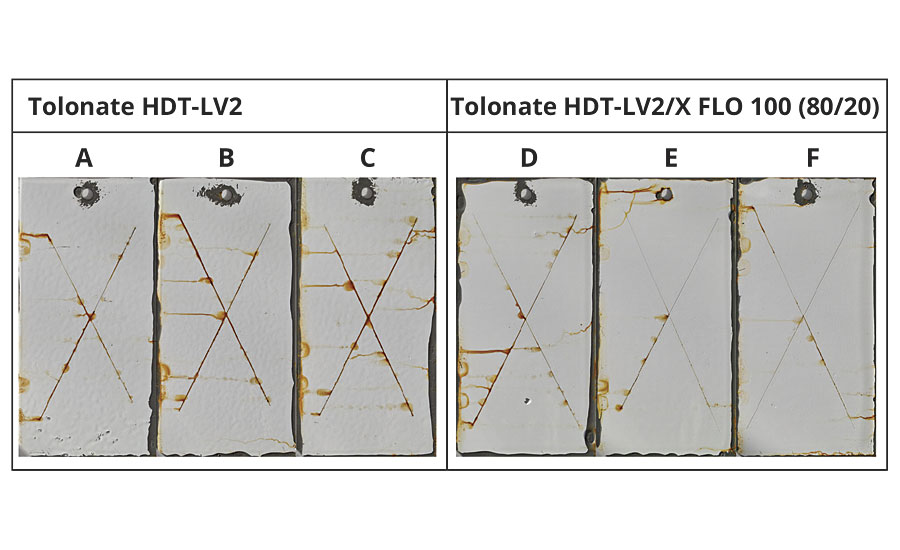
FIGURE 12 » Panels with protective coatings crosslinked with (panels: D, E and F) and without (panels: A, B and C) reactive diluent after 28 days of salt spray exposure.
This result can be interpreted by the atypical molecular structure of the Tolonate X FLO 100 and the presence of apolar hydrocarbon chains. To demonstrate this, we have measured the contact angles with water of various films made of polyaspartic ester resins crosslinked at a stoichiometric ratio of NCO/NH=1 with blends of HDI-based polyisocyanates containing different amounts of reactive diluent (Table 8).
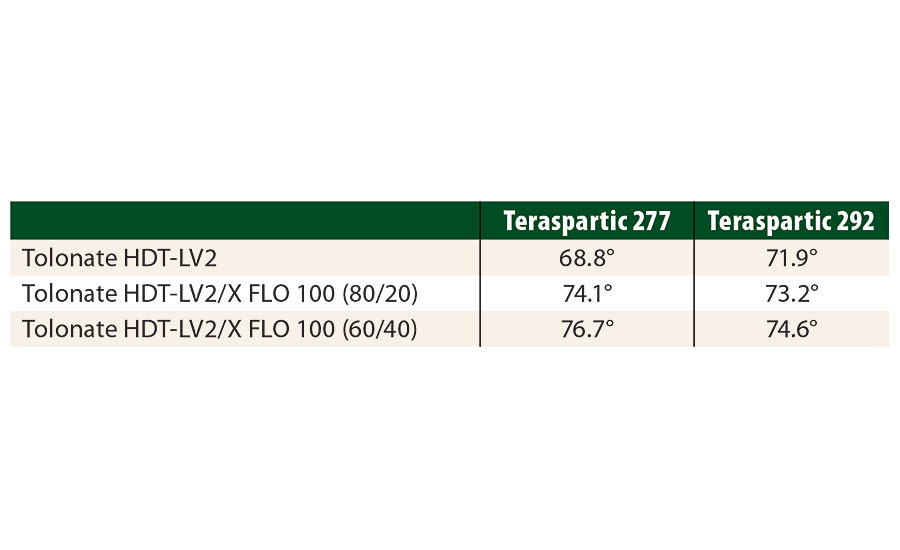
TABLE 8 » Contact angle measurements for films based on polaspartic ester resins crosslinked with HDI-based polyisocyanate blends containing different amounts of the reactive diluent.
The increase of the contact angle clearly shows the surface to be more hydrophobic with increasing amount of Tolonate X FLO 100. This more hydrophobic character of the films containing Tolonate X FLO 100 plays definitely a decisive role in the improvement of the salt spray resistance.
Conclusions
The present study shows that the reactive diluent Tolonate X FLO 100, used in blend with conventional HDI-based polyisocyanates, can bring significant advantages to 2K polyaspartic systems. On one hand, it enables formulators to reduce blend viscosity and to increase pot life. A modeling approach has been used to simulate the effect of the use of the reactive diluent on the gel point, which is directly linked to the pot life. On the other hand, the overall performances of the coating remain unchanged or are improved. In the results found, we could more particularly highlight that the presence of Tolonate X FLO 100 enables the reduction of shrinkage coming from the fast crosslinking mechanism, and consequently contributes in improving adhesion performance. Furthermore, the hydrophobic structure of the molecules enables to improve the corrosion resistance of the coatings.
Acknowledgements
Special thanks to those who contributed to this paper and more specifically Jean-Yves Martin for his involvement in the testing. Philippe Barbeau is also gratefully acknowledged for his support and for making this work possible.
References
1 Zwiener, C.; Sonntag, M.; Kahl, L. Aspartic Acid Esters - A New Line of Reactive Diluents for High-Solids Two-Pack Polyurethane Coatings. Twentieth FATIPEC Congress, (1990) (p. 267).
2 Angleoff, C.; Squiller, E.E.; Best, K. E. Two-Component Aliphatic Polyurea Coatings for High Productivity Applications. Journal of Protective Coatings & Linings (2002), 42-47.
3 Squiller, E.E.; Best, K.E. Polyaspartics for Corrosion Protection Applications. Corrosion and Prevention (2006), (pp. 490-495). Hobart/Autralia.
4 Rasing, R.; Bender, J.D. Beyond Green: A toolbox for uncompromised industrial flooring. Polymers Paint Colour Journal (2013), 24-28.
5 Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers: Gelation. Journal of the American Chemical Society (1941, November), 63(11), pp. 3083-3090.
6 Stockmayer, W.H. Therory of Molecular Size Distribution and Gel Formation in Branched Polymers II. General Cross Linking. Journal of Chemical Physics (1944), 12(4), p. 125.
7 Flory, P.J. Principles of Polymer Chemistry (1953), Ithaca and London: Cornell University Press.
8 Chambon, F.; Winter, H.H. Linear Viscoelasticity at the Gel Point of a Crosslinking PDMS with Imbalanced Stoichiometry. Journal of Rheology (1987), 31, pp. 683-697.
9 Broekaert, M. Polyurea spray coatings. European Coatings Conference. Berlin, (2002).
10 Chambon, F.; Winter, H.H. Stopping of Crosslinking Reaction in a PDMS Polymer at the Gel Point. Polymer Bulletin (1985), 13, pp. 499-503.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!