Finding Formaldehyde-Free Substitutes for Encapsulation

Encapsulation technologies are at the heart of some of today’s most exciting consumer and industrial products, from self-healing paints and coatings to targeted-release agrochemicals. But many of these miracle products are hiding a dirty little secret: formaldehyde.
For most of these products, there is no hard evidence that the levels of formaldehyde they contain present a risk to human health or the environment. However, consumer concerns and regulatory scrutiny are pushing many manufacturers to look for safer alternatives to formaldehyde-based encapsulation technologies. The challenge? Finding replacement encapsulation materials that match the properties that have made formaldehyde resins so popular. Battelle is working to develop formaldehyde-free alternatives using a process we call “Encapsulation by Design.”
Melamine Formaldehyde Resin: A Tough Material to Beat
Melamine formaldehyde resin is one of the most widely used materials for encapsulation. And for good reason: formaldehyde-based resins are cheap, easy to work with and have chemical properties that make them highly useful for encapsulated products.
Encapsulation requires materials that combine two important attributes. First, the capsule must provide a stable and effective barrier between an active ingredient and the outside environment or surrounding material. Second, the capsule must be able to break open or dissolve in response to a specific trigger in order to release the active ingredient at the appropriate time. By manipulating the molecular structure of the shell material, chemists can design capsules that respond to different kinds of triggers.
This process has enabled the development of innovative materials and coatings that are able to respond to their environments to deliver an active ingredient exactly when it is needed. These products include shelf-stable formulations that combine reactive chemical components such as bleaches and activators, healing agents and catalysts, or enzymes and substrates. The chemical components are kept safely separated by the protective polymer shell until a selected trigger (e.g. heat, temperature, dilution, pH or other environmental trigger) breaks or dissolves the shell and releases the active agent. Encapsulation is the technology behind groundbreaking products such as self-healing paints that respond to the presence of corrosion, self-curing resins and time-release personal care products.
Melamine formaldehyde resins have long been used in paints, coatings and laminates because they cure into polymers that are stable, and moisture and chemical resistant. These same properties make formaldehyde-based polymers useful for a broad range of encapsulation applications.1
Melamine formaldehyde polymers form a three-dimensional crosslinked network that resists both hydrophobic and hydrophilic chemicals, including water, organic solvents and acids. While they will degrade over time, they are more stable and longer lasting than most alternatives. Due to their complex crosslinked molecular structure, capsules made using melamine formaldehyde resins provide excellent protection for encapsulated ingredients against both moisture and other chemicals. Their structure also enables the addition of functional groups that provide the trigger mechanism for release of the enclosed material.
These properties have made melamine formaldehyde resins a popular choice for many different kinds of encapsulated products, including agrochemicals, personal care products, and paints and coatings. However, their use has also raised concerns about the potential for formaldehyde exposure.
Rising Concern over Formaldehyde Exposure
The National Toxicology Program, the International Agency for Research on Cancer and the U.S. Environmental Protection Agency (EPA) have classified formaldehyde as a human carcinogen. Specifically, formaldehyde exposure has been associated with myeloid leukemia, lung cancer and nasopharyngeal cancer. Acute exposure effects can include irritation to the eyes, nose, throat, lungs or skin. People can be exposed to formaldehyde by inhaling gasses emitted by formaldehyde-containing materials or absorbing it through the skin in liquid products.
Melamine formaldehyde resins used in coatings, pressboard, and other consumer products and building materials have been shown to outgas formaldehyde over time, generating consumer concerns about exposure risks and prompting many manufacturers to look for low- or no-formaldehyde alternatives. The EPA has set formaldehyde emission limits for wood composite products, but has not yet set standards for other products containing melamine formaldehyde resins.
It is unknown whether encapsulated products using melamine formaldehyde-based polymers present a formaldehyde exposure risk. Few studies have been done to determine how much formaldehyde is released,2 and under what conditions, while encapsulated products are stored or used. The industry also lacks specific toxicology studies on products containing formaldehyde-based capsules. These knowledge gaps make it difficult to determine whether the products truly present a risk to human health or the environment.
However, consumer concerns about formaldehyde exposure make moving to low- or no-formaldehyde formulations a smart move for manufacturers. Finding formaldehyde-free formulations will also help companies prepare for potential regulatory changes in the future.
The Search for Formaldehyde-Free Substitutes
Of course, melamine formaldehyde resin is far from the only material usable for encapsulation. Biobased polymers like cellulose, gums and alginates are already in wide use for many encapsulated products.3-6 These materials are considered to be more environmentally friendly and safer for consumers. Unlike petroleum-based plastic microbeads - which have now been banned due to concerns about their environmental persistence and impact on freshwater organisms - biobased materials break down easily in the environment. Since they are built on natural, Generally Recognized as Safe (GRAS) materials, they are not believed to present risks to human health or the environment.
However, the same qualities that make them so environmentally friendly also make them less effective for many encapsulation applications. Unlike the strong, cross-linked three-dimensional network provided by melamine formaldehyde resins, biobased polymers such as cellulose or alginate have a chemical backbone that is simpler and highly sensitive to moisture and microorganisms. Many of the active ingredients and solvents used with formaldehyde-based polymers are not compatible with existing biobased materials. In addition, biobased materials may not provide enough protection in environments where moisture and microorganisms are a problem.
More work is needed to expand the range of options for formaldehyde-free encapsulation materials. Battelle is working to develop new encapsulation materials that can be used with a broader range of active materials and can respond to a variety of different triggers.
Encapsulation by Design
There is no “one-size-fits-all” solution for encapsulation. While the properties of melamine formaldehyde resins make them applicable for a broad range of applications, replacing them with formaldehyde-free alternatives is likely to require a suite of different product solutions. These will include variations on existing alginate and cellulose materials, but industry is likely to need other options for applications where these materials do not provide the right chemical properties.
The choice of shell materials for encapsulation depends in several factors, including:
- The physiochemical properties of the active ingredient it will encapsulate;
- The chemical and physical properties of the environment surrounding the capsule;
- The desired particle size; and
- The desired release mechanism.
Materials can be encapsulated by several methods, including spray drying/congealing, phase separation, solvent evaporation and coating. Battelle uses a particle-forming polymerization approach to encapsulate both solid and liquid active ingredients. In this process, active ingredients are suspended in a medium using a stabilizer along with a shell-forming monomer and an initiator. Polymerization occurs in the solution as the monomer and initiator react. The active ingredient acts as a nucleus for polymer growth, resulting in a spherical polymer shell forming around an active ingredient core (Figure 1). Typically, the product is then isolated by filtration.
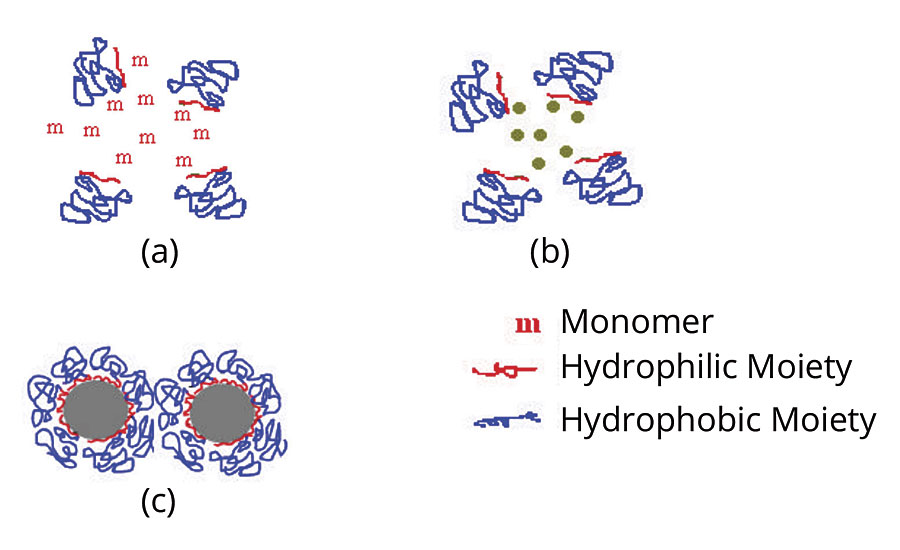
FIGURE 1 » Mechanism of particle formation (a) homogeneous polymerization medium (b) nucleation stage and (c) stabilization of polymeric particles by the added stabilizer.
A variety of monomers can be used in this process, depending on the required characteristics of the resulting polymer shell and the trigger mechanism. The trigger mechanism is often added during polymerization as well, by introducing functional monomers that are responsive to specific triggers. In cases where the active enclosed by the capsule and the stimuli-responsive functional monomer are not compatible, the functional groups are attached to the shell in a post-polymerization process.
Shells can be designed to respond to a variety of different triggers, including pressure, temperature, pH level, dilution, UV light, or exposure to specific chemical or biological signals. The final polymer must be compatible with both the active ingredient that it encloses and the solvent or environment in which the capsule will be stored.
Bringing Formaldehyde-Free Encapsulation Products to Market
Battelle has identified GRAS materials that have the potential to replace formaldehyde-based polymers for the encapsulation of wide range of active materials (Figure 2).
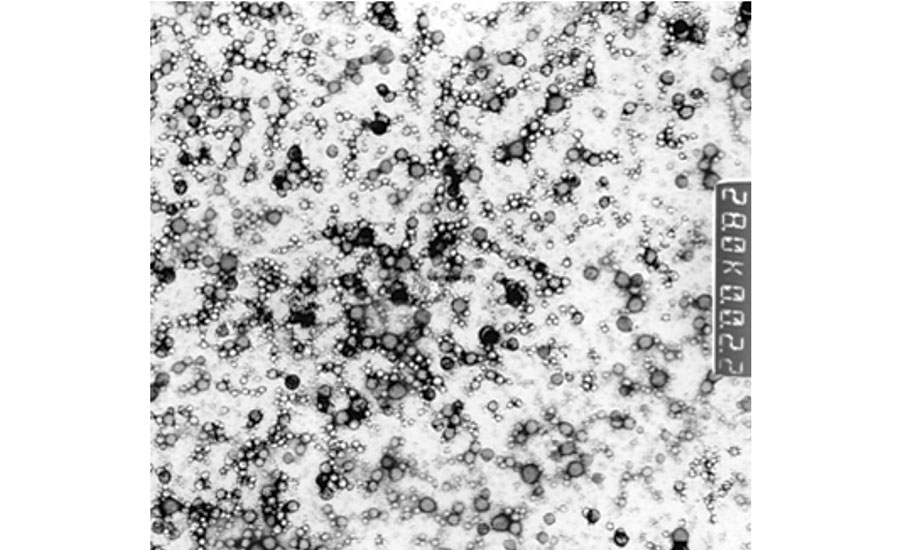
FIGURE 2a » Transmission Electron Microscopy of polymer particles filled with liquid active ingredient.

FIGURE 2b » Scanning Electron Microscopy of polymer particles filled with solid active ingredient.
Starting with GRAS materials, we can design encapsulation alternatives that will be safer and more environmentally friendly than their formaldehyde-based cousins. Of course, the toxicity of the final product depends on the characteristics of the active ingredients as well as the way the starting material is changed in the encapsulation process. For example, alginate is widely studied and considered safe for use as a food additive and in cosmetics, but alginate modified with polymers used for encapsulation may have different properties due to the polymerization process, the addition of functional groups or reactions with active ingredients.
While we cannot claim the new materials are nontoxic without further study, we can surmise that they are likely to be less toxic than materials containing formaldehyde. For example, we can ensure that the selected starting material is not a known endocrine disruptor or carcinogen. Battelle is currently conducting toxicology studies on some of the most promising materials for formaldehyde-free encapsulation.
Adding formaldehyde-free alternatives to the encapsulation arsenal will be beneficial for many manufacturers, especially those creating products used directly by consumers or in building materials used in residential or office buildings. It will also reduce exposure concerns for workers involved in manufacturing encapsulated materials or using paints, coatings or resins that contain encapsulated ingredients.
To bring these safer materials to market, more work needs to be done to design materials for specific applications that retain the positive characteristics of melamine formaldehyde resin without the drawbacks. With the right chemistry, the industry will be able to meet the challenge.
References
1 Mishra, M. Editor, Handbook of Encapsulation and Controlled Release. CRC Press: 2016; p 1511.
2 Paiva, N.T.; Henriques, A.; Cruz, P.; Ferra, J.M.; Carvalho, L.H.; Magalhães, F.D. Production of Melamine Fortified Urea-Formaldehyde Resins with Low Formaldehyde Emission. Journal of Applied Polymer Science 2012, 124 (3), 2311-2317.
3 Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a Delivery Vehicle for the Controlled Release of Bioactive Molecules. Journal of Controlled Release 2005, 109 (1), 256-274.
4 Imam, S.H.; Bilbao-Sainz, C.; Chiou, B.S.; Glenn, G.M.; Orts, W.J. Biobased Adhesives, Gums, Emulsions, and Binders: Current Trends and Future Prospects. Journal of Adhesion Science and Technology 2013, 27 (18-19), 1972-1997.
5 Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation Using Starch as Wall Material: A Review. Food Reviews International 2016, 1-14.
6 Schnur, J.M.; Price, R.; Rudolph, A.S. Biologically Engineered Microstructures: Controlled Release Applications. Journal of Controlled Release 1994, 28 (1), 3-13.
You can watch a webinar about formaldehyde-free substitutes for encapsulation at www.pcimag.com/webinars. The webinar was presented at Coatings Trends & Technologies 2017.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









