Positive Tactile Interaction Coatings
Soft Materials Based on Silicones and Siloxane-Polyol Urethanes

While the sensory appeal of coatings has always been an important driver of consumer acceptance of devices and appliances, positive tactile interaction properties of coatings are gaining increased attention in an industry that has focused primarily on their optical characteristics. Understanding both the aesthetic and functional significance of these tactile characteristics is therefore an area of opportunity. Physical human contact with a surface can have positive and negative attributes; positive attributes are often associated with terms such as “good-feel” “soft touch,” “lubricious,” “warm,” “cushion,” and “slick,” but are only meaningful in the context of a specific application. For example, “slip” may be a positive attribute in one application, but negative in another. Direct contact with metal, cellulosic or polymeric substrates that are generally experienced as dull, or even unpleasant, can be transformed into an interaction that is functionally pleasant, or even luxurious, when mediated by a coating that stimulates the mechanical and thermal receptors of the skin differently. Applications that rely on positive tactile interaction with coatings include healthcare appliances, mobile devices, automotive interiors, synthetic leather, and, in a broad sense, textile finishes as well as color and hair-care cosmetics. Negative tactile interactions may simply result in touch-wear failure of a coating; in textile coatings, they may be associated with friction and modulus properties that cause decubitus ulcers. Finally, the issue of tactile properties has become critical in the market acceptance of interactive devices ranging from hand-held devices to human interfaces with robotics.
Figure 1 shows a variety of applications where tactile interactions combine with functional properties. Synthetic leather finishes require control of modulus and coefficient of friction. Capacitive arrays for fingerprint recognition require a combination of tactile interaction, dielectric properties, and permeability of gases and moisture. Oxygen permeability, hydrophilicity and mechanical properties in siloxane-urethane copolymers utilized in contact lenses demonstrate extreme control in the medical arena that can be translated from bulk molding to thick films.
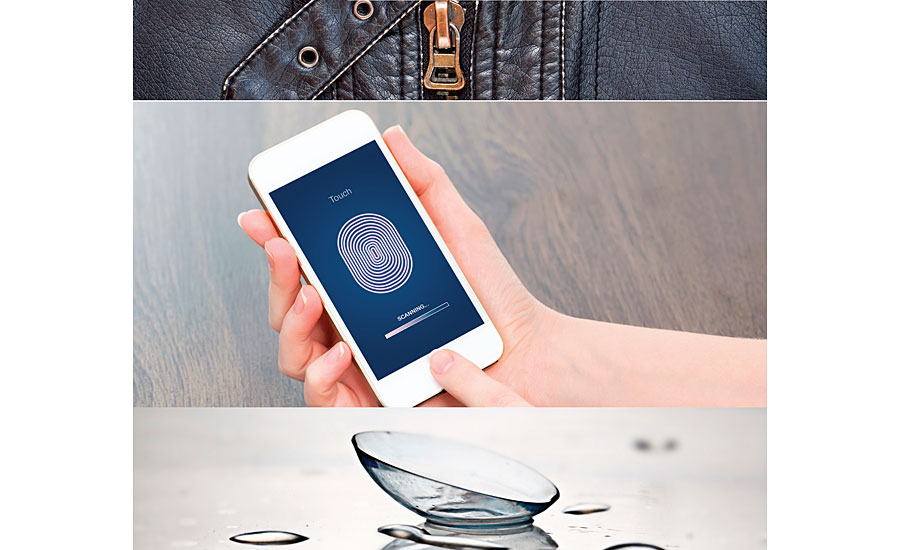
FIGURE 1 » Applications where tactile properties are important
The sensory appeal of a given coating is defined at a distance by its positive visual attributes. However, when personal contact involving touch and motion is an intrinsic consideration, the acceptance of a coating depends on positive tactile interaction. The interaction by a combination of touch and motion with an object can be defined as “haptic interaction.”
While soft materials such as vinyl plastisols and polyurethanes dominate the field of positive tactile interaction coatings as a whole, silicones and siloxane-modified urethanes dominate in areas where the precision of interaction is critical. Silicones and siloxane-modified urethanes are well established in thermoset and thermoplastic embodiments. This is particularly true in the medical arena, where they have been used to fabricate devices such as catheters, pacemaker leads and artificial heart valves. While biocompatibility is the salient property associated with these materials, they often provide positive tactile interactions. Measureable physical properties associated with both the success of these materials and positive tactile interaction include hydrophobicity, flexibility, low surface energy, and coefficient of friction (both dry and hydrodynamic). Until now, these established properties have not been widely translated to coating applications. This is in part because the formulation of silicone-modified polyurethanes has been handicapped by a lack of appropriate siloxane polyols, which have been too high in molecular weight or too polydisperse to achieve acceptable film mechanical properties. Further, low-molecular-weight contaminants intrinsic to conventional equilibrium polymerization silicones (volatile cyclics) often interfere with film formation and substrate adhesion. However, recent developments in the controlled polymerization of polysiloxanes have enabled the production of siloxane polyols with precise molecular structures, which can then be reacted to form both silicones and urethanes suitable for positive tactile interaction coatings. The new urethane materials fall into two classes depending on the siloxane polyol used: telechelic siloxane polymers (two termini with the same functionality), and siloxane macromers (oligomeric materials with functionality on one terminus). The specific position and number of hydroxyl groups in these materials enable the introduction of siloxanes into urethanes as segments, terminating blocks and, uniquely, pendant groups.
Figure 2 compares the structures of telechelic and macromeric siloxanes. Compared to conventional urethanes derived from polyether polyols, urethanes derived from siloxane polyols demonstrate improved performance in several areas - including optical, dielectric, UV stability, coefficient of friction, nonstick/release, low modulus and hydrophobicity (Table 1).
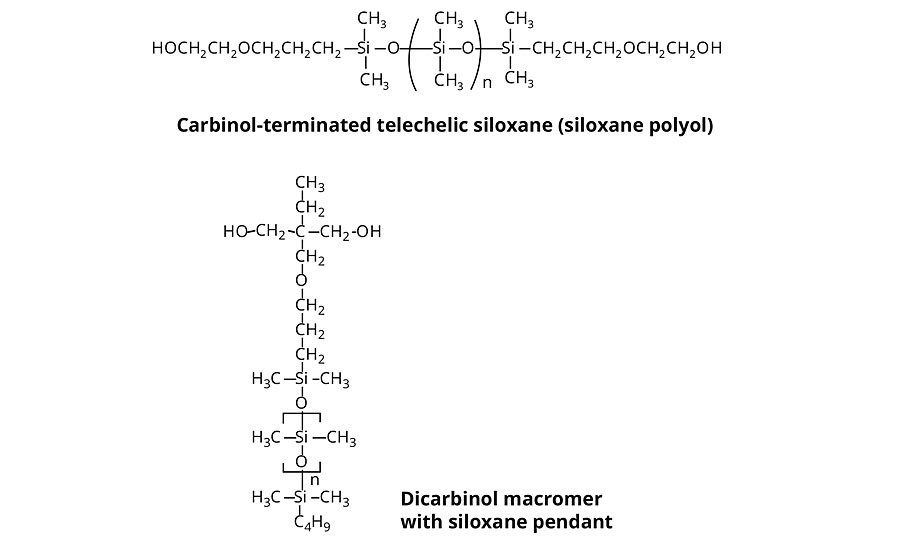
FIGURE 2 » Telechelic and macromeric siloxane structures
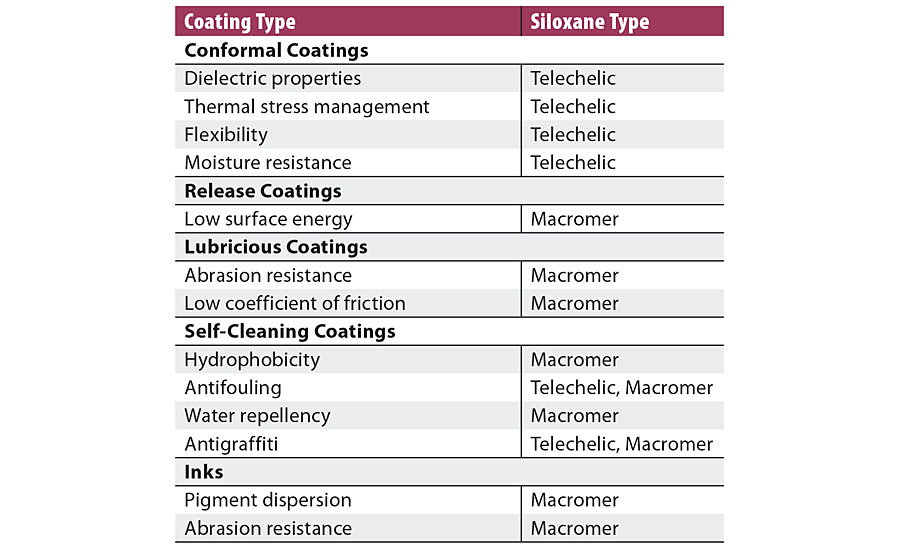
TABLE 1 » Urethane coating systems benefitting from incorporation of siloxane polyols
Human perception of tactile contact is complex, but frequently focused on coefficient of friction, nonstick/slip, modulus and relative hydrophobicity. While positive tactile interaction materials are usually not intended to duplicate skin, it is essential to understand the basic structure and properties of skin as a benchmark for our interaction with these materials. Skin’s physical properties vary depending on numerous factors such as location (on the body), age, gender and hydration. Broadly speaking, skin is incompressible, anisotropic and nonlinear viscoelastic in the normal range of deformation associated with tactile interaction. Figure 3 provides an illustration of skin structure, while Table 2 summarizes the physical properties (typical average values) of skin with data primarily selected from hand and forearm areas.

FIGURE 3 » Illustration of skin structure
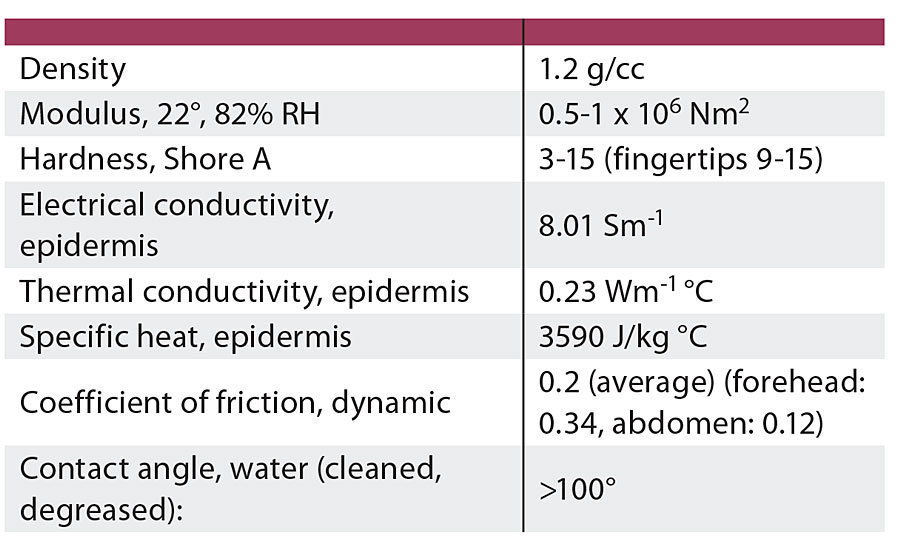
TABLE 2 » Selected typical properties of skin
There are two major classes of coatings associated with positive tactile interaction in which the benefits of siloxane-polyol-derived urethanes are enabling new applications. Both of these classes’ physical properties are broadly similar to those of skin. In the first class, indirect - e.g., textural or capacitive - interaction with the substrate is essential. A capacitive interaction is exemplified in fingerprint recognition by submicron-scale capacitive arrays that are coated for protection but remain responsive to fingertip structure; these are generally thin coatings (<250 microns), which is often referred to as “conformal” coatings in electronic applications. In the second class of coatings, a specific resistance, defined by use parameters, to physical displacement - e.g., indentation or frictional displacement - is desired. These coatings are generally >1000 microns in thickness. Apart from the intrinsic properties of the coating material, other factors such as coating thickness and the ratio modulus of the coating to the modulus of the substrate must be considered. In general, thin-film or conformal coatings have high siloxane content that is incorporated into the urethane as segments, while thick-film or bulk coatings have lower siloxane content that is incorporated as pendants.
Thin-Film or Conformal Siloxane-Modified Urethanes
When either the structural features or texture of a substrate must remain palpable, the conformality of the chosen coating becomes essential. Thin-film or conformal silicone coatings rely on the flexibility of the silicone backbone (Figure 4). For example, polydimethyl-siloxane is thermodynamically driven to spread and form monomolecular films, since its surface tension (20.4 mN/m) is below the critical surface tension of solid or constrained films (22.7 mN/m). The higher the silicone content, the greater the tendency to form conformal films. For polymers incorporating telechelic siloxanes as blocks, it has been observed that flexibility is limited when there are less than 6-8 siloxane units in the blocks. As a result, most formulations use a minimum of 10 siloxane units to introduce conformality into coatings. Table 3a summarizes the commercially available hydroxyl-terminated siloxanes. In polyurethane technology, “polyols” typically refers to molecular species with two or more hydroxyl groups. Tabular information here utilizes the term “carbinols” to refer to hydroxyl groups attached to a carbon atom. This differentiates the chemistry from hydroxyl groups attached directly to a silicon atom, termed “silanols,” which do not form hydrolytically stable urethanes.
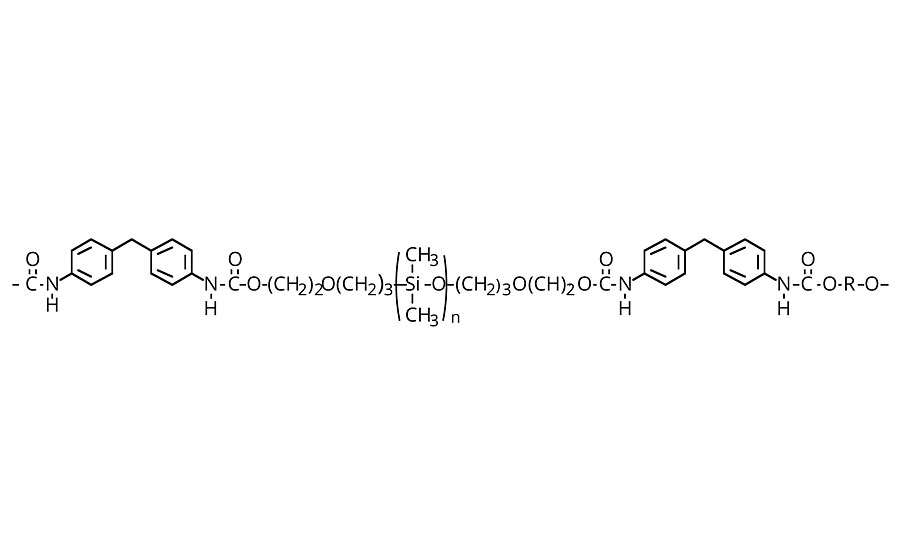
FIGURE 4 » Thin-film siloxane-modified urethane
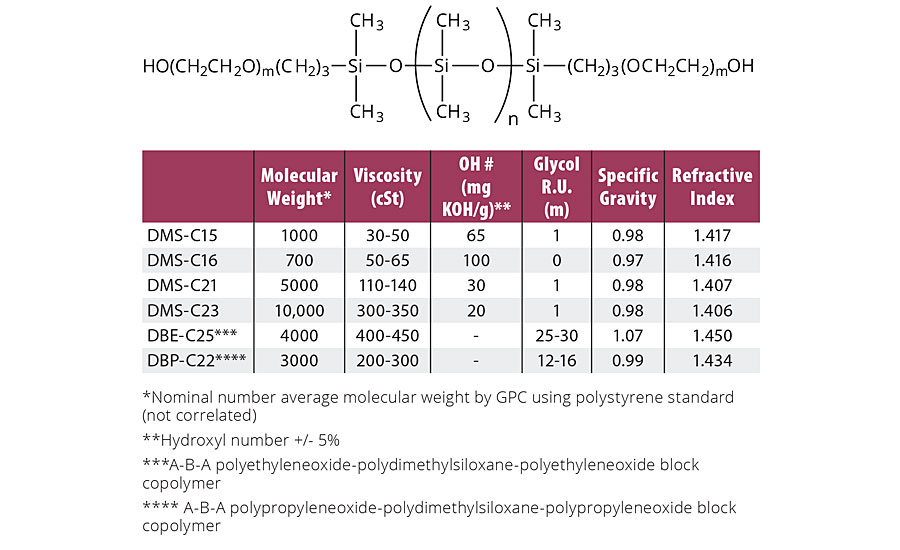
TABLE 3a » Properties of carbinol (hydroxyl)-terminated siloxanes
The selection of a particular telechelic depends on a combination of mechanical property requirements, conformality, stain resistance and lubricity. In general, conformality and stain resistance increase with siloxane block size, while mechanical properties decrease. Other factors complicate this simple trade-off, however. For example, if lubricity requires a hydrodynamic water interface, a higher content of poly(ethylene glycol) (PEG) content is desirable. On the other hand, a large siloxane block with little or no PEG is desirable if low modulus and minimal slip associated with tactile positioning is required. These considerations are further complicated by additional use requirements such as coefficient of friction: while the relative coefficient of friction must always be considered, the specific application dictates consideration of more complex frictional variables as well. If the static coefficient is higher than the dynamic coefficient of friction, the material will exhibit nonstick-slip properties. More commonly, materials will exhibit higher dynamic coefficient of frictions higher than static, leading to the exhibition of stick-slip properties. Water is also an ever-present consideration. In the case of skin against cotton fabric, the coefficient of friction more than doubles as the fabric becomes wet from 0.42 to 0.91. In other cases, water provides a hydrodynamic lubricating layer. While slip may be desirable in some applications, braking characteristics may be more important in others (in order to achieve braking characteristics, the CoF should exceed 1). Table 3b summarizes commercially available siloxanes with pendant hydroxyl groups.
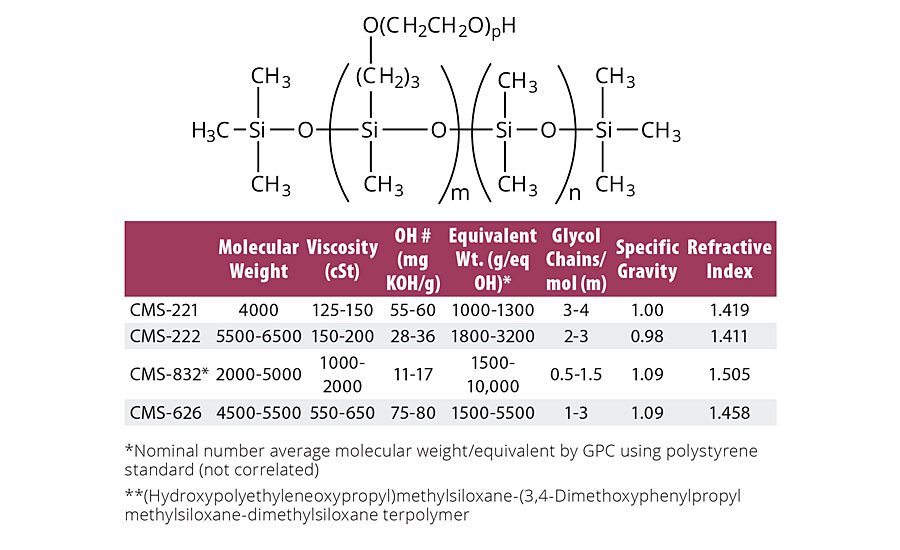
TABLE 3b » Properties of carbinol (hydroxyl) pendant siloxanes
Thick-Film Siloxane-Modified Urethanes
In general, thick-film coatings mask or alter the underlying texture or structure of substrates. Surface tactile interaction is not discounted in these applications, but the hardness, modulus and elasticity of the film become more important. Since their mechanical strength is much greater than that of polysiloxanes, the general formulation strategy for polyurethanes is to maintain an organic urethane backbone and introduce siloxanes as pendant groups (Figure 5). Due to the lower surface energy of trimethylsiloxy end groups compared to the dimethylsiloxy groups that comprise the backbone of the telechelics, the use of pendants significantly increases water repellency. Because siloxane macromers are relatively expensive, the organic polyol content of a standard formulation is generally substituted with the minimal amount of siloxane macromers to provide the desired effect: typically stain release or coefficient of friction. Synthetic leather finishes, which use a silicone macromer and isophorone diisocyanate (IPDI) in combination with conventional polyols, offer a successful example of this approach.
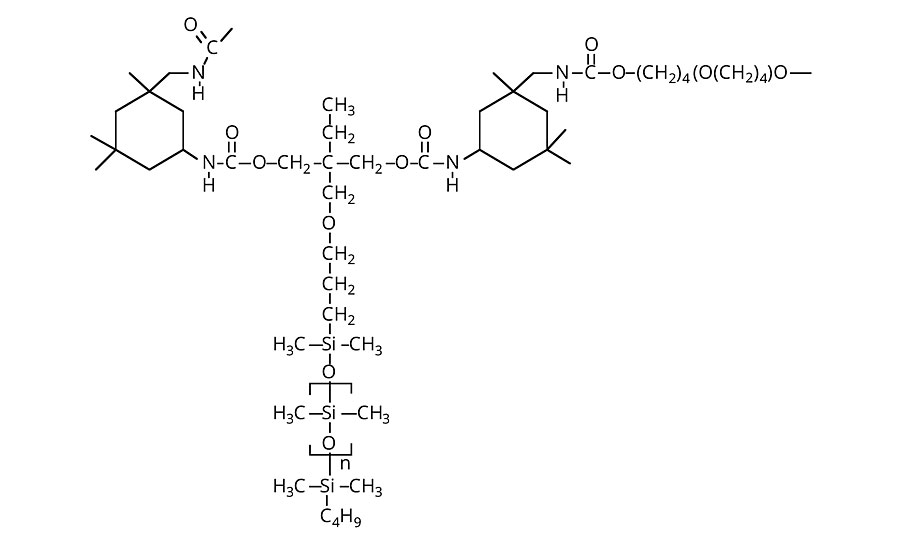
Figure 5 » Thick-film siloxane-modified urethane
Table 4 compares IPDI-based urethanes that were modified by addition of: (A) a nonfunctional polydimethylsiloxane [DMS-T15]; (B) a carbinol-terminated telechelic silicone incorporated in the polyurethane to form a block copolymer (DMS-C21); and (C) a dicarbinol-terminated macromer polymerized into the resin to introduce pendant siloxanes (MCR-C62). The silicone content was 3.0-3.5% in all cases. While the additive approach increases contact angle, indicating an increase in hydrophobicity and release, the effect reduced with abrasion. The block polymer formed from the telechelic showed a moderate increase in contact angle that was maintained after abrasion, but the coefficient of friction was higher and abrasion resistance was not improved. The polymer formed from the macromer demonstrated increase in hydrophobicity, decreased coefficient of friction, and a significant increase in abrasion resistance. The results may be explained by maintenance of a nonsiloxane organic polymer backbone and the free-rotational energy of a siloxane attached at only one end to the polymer backbone.
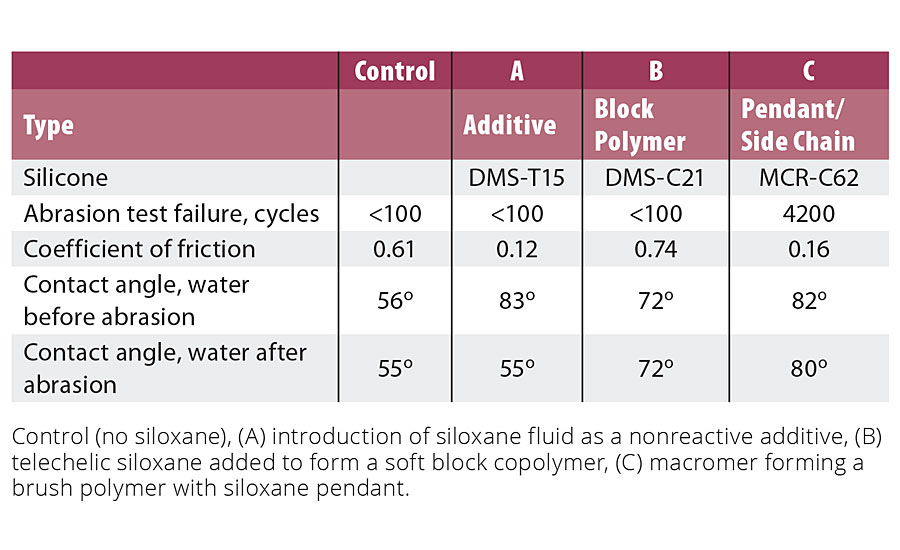
TABLE 4 » Comparing contact angle, abrasion and friction in an IPDI-based urethane
Apart from friction and release properties, the benefits of incorporating siloxanes as soft blocks into urethane polymer structures are often associated with the reduction of hardness and modulus to a range closer to that of human soft tissues. Formulation considerations are more complex, and optimization of properties depends on the type and size of the siloxane block. Further, they are frequently used in combination with other soft block materials such as poly(tetramethylene oxide) and aliphatic polycarbonatediols. It is unusual on a weight percent basis for the siloxane content to exceed 20%. At higher levels the loss of tensile strength and other mechanical properties is usually unacceptable. Table 5 provides mechanical properties of a range of siloxane urethane systems.
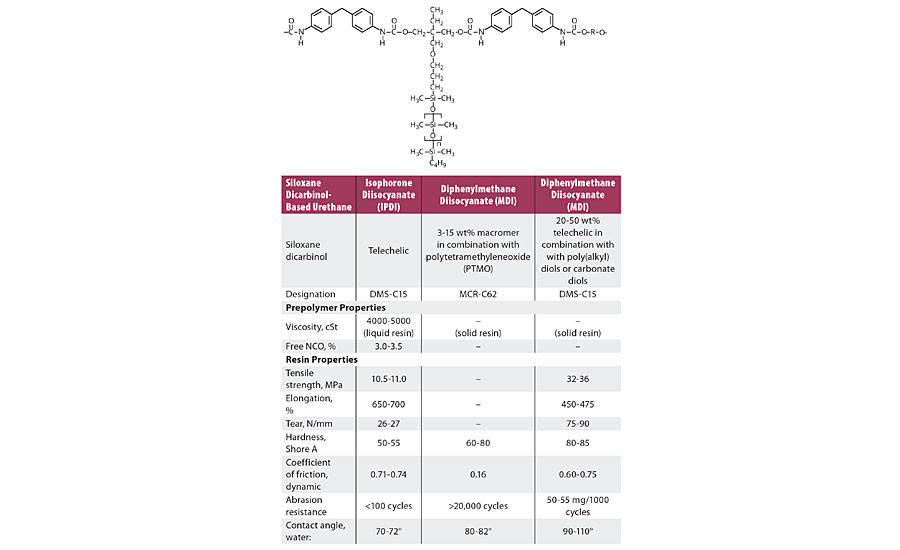
TABLE 5 » Properties of siloxane polyol-modified urethanes
Thick-Film Silicone Coatings
Depending on the formulation, silicone coatings elicit a wide range of tactile response. If they contained low-molecular-weight, particularly volatile species, they are associated with a “silky” feel and exhibit slip. On the other hand, silicone coatings that have been depleted of low-molecular-weight species are associated with a “tacky” feel. Common to both these experiences is the extreme hydrophobicity of the polymer. When silicone coatings are free of low-molecular-weight species, they exhibit high coefficients of friction and, due to their relatively low mechanical properties, failure by abrasive and adhesive spalling during continuous tactile interaction. Well-defined silicones with a central vinyl functionality, discrete PEG2 (MCS-VX15), PEG3 (MCS-VX16), or tetrahydrofurfuryl (THF) (MCS-VF14) pendant end groups can be used as comonomers in addition-cure, platinum-catalyzed two-part silicone elastomer formulations to introduce hydrophilicity (Figure 6). In such formulations, the surface tribological properties are modified by introducing a hydrodynamic lubricating layer of adsorbed water. The modified silicone elastomers retain optical clarity and mechanical performance characteristic of this class of material with up to 15 wt% comonomer in the two-part formulation. Contact angle measurements of deionized water on the silicone elastomer surface showed improved wettability with comonomer content: the elastomer surface shifts from hydrophobic (contact angle ~120°) to hydrophilic (contact angle < 90°) at ~8 wt.% comonomer (Figure 7a). Coefficient of friction measurements of the modified silicone elastomers demonstrate increased surface lubricity with comonomer loadings (Figure 7b).
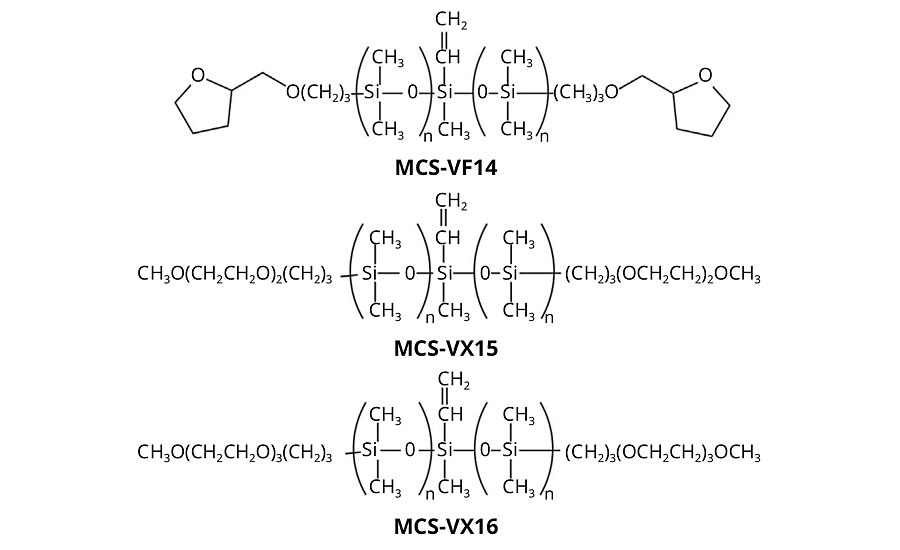
Figure 6 » Thick-film silicone structures
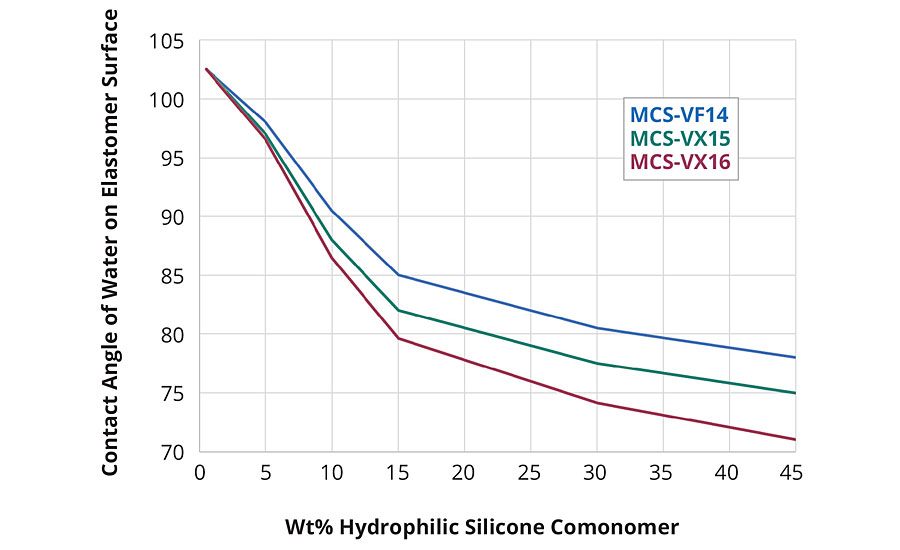
Figure 7a » Effect of comonomer on contact angle and hydrophobicity of silicones
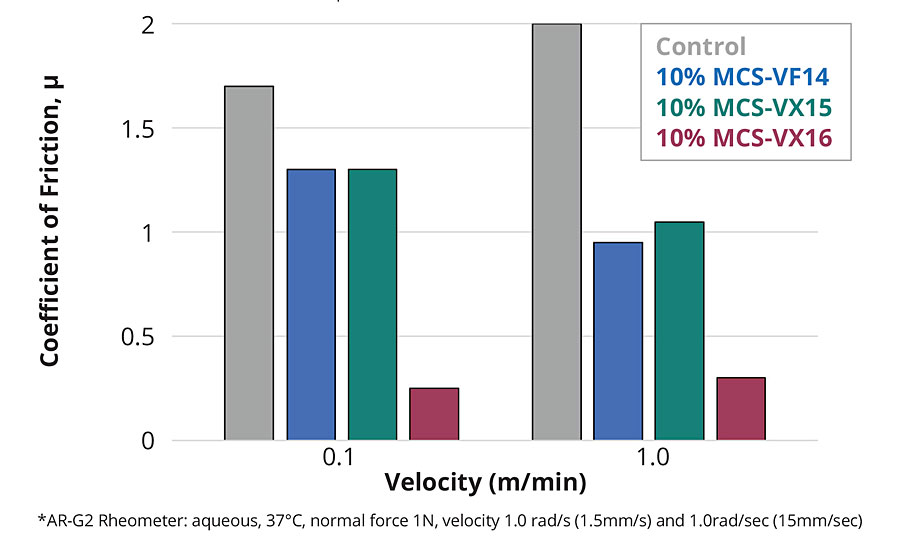
Figure 7b » Effect of comonomer on coefficient of friction of silicone elastomer surface in an aqueous environment
Conclusion
Applications for tactile coatings that require specific biointerface response will continue to grow in demand and in complexity of performance specifications. In the area of response-critical soft interactions, both urethane- and silicone-based materials will dominate. Reactive siloxane telechelic and macromeric siloxanes with their intrinsic physical properties and control of structure are now able to extend the range of performance for both urethane- and silicone-based positive tactile interaction coatings.
References
1. Cua, A.; Wilhelm, K.; Maibach, H. British Journal of Dermatology, 123(4), 473, 1990.
2. Ginn, M.; Noyes, C.; Jungermann, E. J. Colloid and Interface Science, 26(2), 146, 1968.
3. Ward, R.S.; Jones, R.L. Comprehensive Biomaterials, 1, 431-477, 2011.
4. Goff, J.; Arkles, B.; Sulaiman, S. Materials Res. Soc, Proc., 1626, mrsf13-1626-k11-07, 2014.
5. Kozakiewicz, J. Progress in Organic Coatings, 27, 123, 1996.
6. Arkles, B.; Kimble, E.; Goff, J. Reactive Silicones: Forging New Polymer Links, 2016, Gelest Inc.
7. Loomis, J.M.; Lederman, S.J. Handbook of Perception and Human Performance. Ed., Boff, K.R.; Kaufman, L.; Thomas, J.P. 2(1986): 31-40.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









