Emulsion Polymerization of Hydrophobic Monomers
Using Diphenyl Oxide Disulfonate Anionic Surfactant

The effective transportation of monomer from monomer droplets through the water phase into particles is a crucial step in the emulsion polymerization of hydrophobic monomers with very low water solubility, such as VeoVa™ 10 monomer (vinyl ester of neodecanoic acid) or isodecyl methacrylate (IDMA).
In previous work,1,2 we have demonstrated that Calfax® 16L-35 (sodium hexadecyl diphenyl oxide disulfonate) has excellent association with hydrophobic monomers and acts as an effective monomer transporter from monomer droplets through the water phase to latex particles. Using Calfax 16L-35, a conventional emulsion polymerization process with an ammonium persulfate initiator can produce homopolymers of VeoVa 10 monomer and IDMA as well as the copolymers or terpolymers of these monomers with other monomers such as styrene, MMA and TBMA.
In this article, we build on the previous work by investigating the effects of pre-emulsion agitation and the incorporation of acid monomers on the emulsion polymerization of VeoVa 10 monomer and IDMA using Calfax 16L-35 as a surfactant. In addition, we performed differential scanning calorimetry (DSC) characterization of some copolymer systems to provide an early indication of how these hydrophobic monomers incorporate in copolymerization with themselves or with commonly used monomers.
Experimental Setup
Our study explored both the effects of agitation during pre-emulsion mixing and the incorporation of acid monomers into very hydrophobic emulsion polymerization systems.
Effects of Agitation During Pre-Emulsion Mixing
To explore the effects of agitation during pre-emulsion mixing, we used a 50/50 mixture of IDMA and VeoVa 10. Both of these monomers are extremely hydrophobic compared to monomers currently used in commercial emulsion polymerization processes.
Since acid monomers are commonly used in emulsion polymerization systems to increase adhesion and stability, we incorporated acrylic acid (AA) or methacrylic acid (MAA) into some IDMA/VeoVa 10 copolymerization trials. AA and MAA were incorporated at 2.0 or 0.5 parts per hundred monomers (pphm), with the acid monomers replacing equivalent parts of VeoVa 10 monomer.
We also revisited the effect of pre-emulsion agitation in the homopolymerization of IDMA and TBMA.
In our conversion assessment and monomer composition calculations for IDMA/VeoVa 10 copolymerization, we assumed a monomer activity of 98.5%3 for IDMA. Nonreactive materials in IDMA were treated as nonvolatile materials that contributed to the solids of the latex.
Incorporation of Acid Monomer
In order to better observe the interaction of a very hydrophobic monomer and a very hydrophilic acid monomer in an emulsion polymerization system, we ran several experiments using a mixture of VeoVa 10 with small amounts of maleic acid (MA). MA was chosen because it is a diacid with much higher water solubility than AA or MAA, allowing us to create a greater contrast with the hydrophobic VeoVa 10 monomer.
For these experiments, maleic anhydride was hydrated to become maleic acid. The mass balance of this hydration reaction was taken into account in calculating the monomer composition as well as the theoretical solids.
Emulsion Polymerization Protocol
The emulsion polymerization experiments were carried out in a 1-liter jacketed glass reactor with four built-in baffles. The agitation of the reaction mixture was conducted using a stirrer system including a polytetrafluoroethylene (PTFE)-covered stainless steel shaft and two 64-mm turbine PTFE blades. The temperature was regulated by an external circulating water bath with heating and cooling capability. The reactor was fitted with a thermometer and feed lines. Both monomer pre-emulsion and initiator solution were fed via FMI pumps above the surface of the reaction mixture. All the monomers and other materials were used as supplied without purification.
Pre-emulsion preparation:
- Mixing speed: 400, 500, 600 rpm
- Mixing time: 25, 45 min
Emulsion polymerization process:
- In-situ seed with 2% pre-emulsion in initial charge, reacted for 5 min
- Reaction temperature: 80 ± 1 °C
- Pre-emulsion feed time: 3.0 hrs
- Initiator solution feed time: 3.5 hrs
- Hold time after initiator feed: 0.5 hr
Other parameters:
- For simplicity of the experiments, no post-additions (neutralizer and biocide) were made.
- Target solids: 45 ± 1%
- Target conversion >97%. Conversion in this work is operationally defined as (measured solids/theoretical solids) x 100.
- Surfactant split and level: varied
- Initiator: ammonium persulfate at 0.1+0.4 = 0.5 pphm
- Buffer: sodium bicarbonate at 0 or 0.3 pphm
- Defoamer: Foamaster111 at 0 or 0.1 pphm
Experimental evaluation:
- Fouling (by visual assessment)
- Grit level (ppm, milligrams of grit per kg of latex), target <500 ppm
- Solids (by gravity method, avg. of triplicate)
- pH
- Latex viscosity (by Brookfield, RV #2@20)
- Particle size and zeta potential (by Zetatrac; see below)
Both particle size and zeta potential of the latex are measured by a Zetatrac, a dynamic light-scattering particle size analyzer with additional hardware capable of measuring the mobility of particles under an electric field.
Other details of the materials used and the emulsion polymerization protocol can be found in our earlier publication.1,2
Thermal Analysis
In order to determine whether the copolymerization of IDMA and VeoVa 10 resulted in random copolymers, the glass transition temperature of several samples was analyzed using DSC. The samples were prepared by casting a thin film on polyethylene sheeting and allowing the films to air dry at room temperature and humidity for a minimum of three days prior to testing. Samples weighing approximately 10 mg were analyzed by DSC using a TA Instruments Q2000 DSC calibrated at 20 °C/min with a traceable Indium standard. The samples were heated from -85 °C to 170 °C at 20 °C/min. The samples were then cooled back to -85 °C at 20 °C/min and were reheated a second time to 170 °C. The purge gas was nitrogen at a flow rate of 50 cc/min. The midpoints of the transition region in the second heating were taken as the glass transition temperature.
Results
Copolymerization of IDMA and VeoVa 10
Results of the copolymerization of IDMA and VeoVa 10 monomer at different pre-emulsion agitation speeds are shown in Table 1, Table 2 and Table 3. The experimental parameters are provided in the tables along with the results of latex properties and the processing evaluation of each run. The calculated glass transition temperatures (Tg) of the copolymers, based on the data provided by the literature using the Fox equation, were also included in the tables for reference.
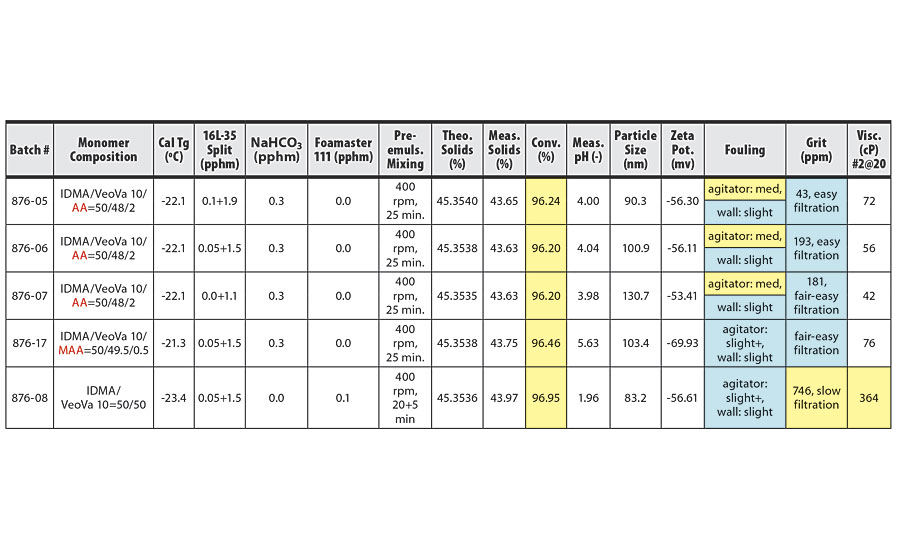
TABLE 1 » Copolymerization of IDMA and VeoVa 10 – pre-emulsion mixing at 400 rpm.
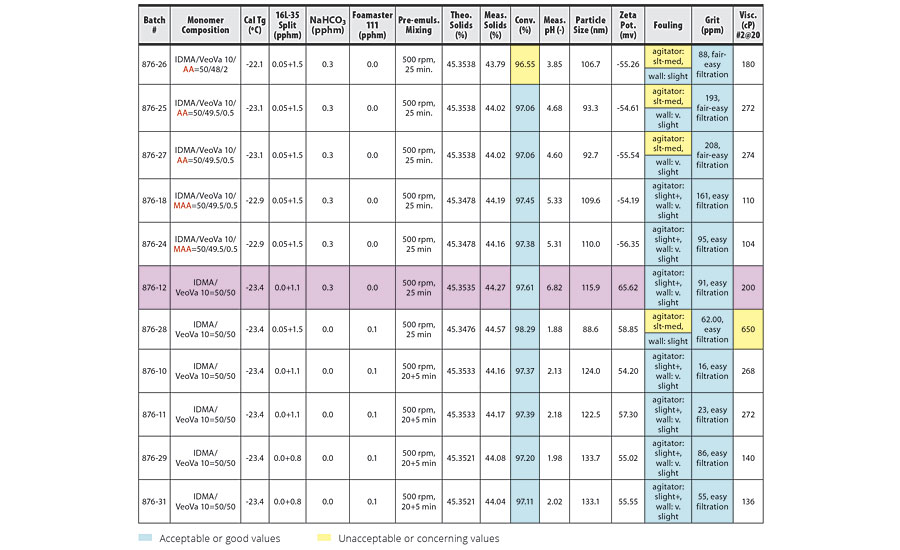
TABLE 2 » Copolymerization of IDMA and VeoVa 10 – pre-emulsion mixing at 500 rpm.
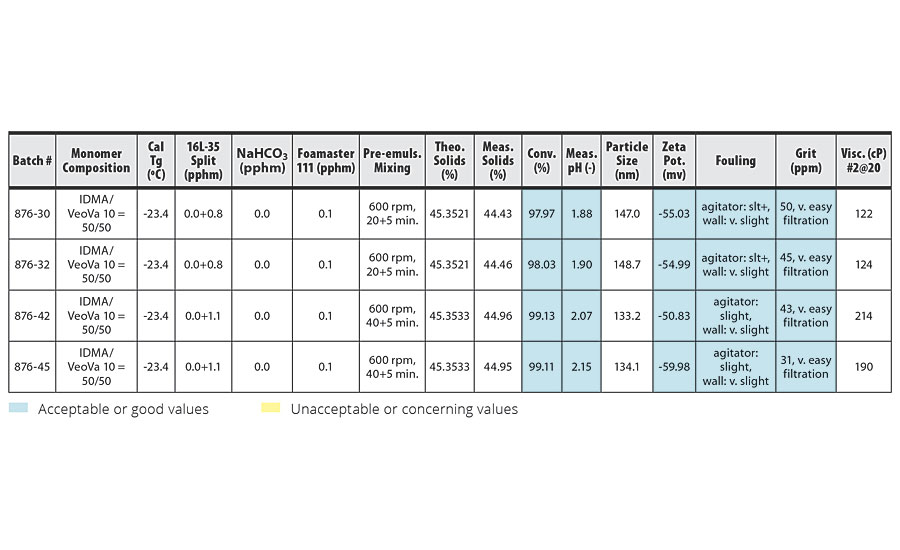
TABLE 3 » Copolymerization of IDMA and VeoVa 10 – pre-emulsion mixing at 600 rpm.
The conversion, grit, fouling and viscosity columns of the tables have been color coded based on whether the trial conditions yielded acceptable or unacceptable results. Blue boxes have acceptable or good values. Yellow boxes have unacceptable or concerning values. In addition, some trials produced a pink-colored latex. These rows have been highlighted in pink in the tables.
For IDMA/VeoVa 10 copolymerization batches with pre-emulsion mixing time of 25 min, increasing mixing speed from 400 rpm to 500 rpm resulted in higher conversion and improved reactor cleanliness. Increasing mixing speed from 500 rpm to 600 rpm produced further improvement in conversion, but no additional improvement in reactor cleanliness. In general, poor reactor fouling was observed at particle sizes of 100 nm or smaller.
These results are clearly illustrated in the comparison of batches 876-29 and 876-31 (Table 2) to batches 876-30 and 876-32 (Table 3). The two batches agitated at 600 rpm show improved conversion over the batches agitated at 500 rpm, with good reactor cleanliness at both 500 rpm and 600 rpm.
The best results were obtained in batches 876-42 and 876-45 (Table 3). Here, pre-emulsion agitation of 600 rpm for 45 min for the IDMA/VeoVa 10 = 50/50 copolymerization using 1.1 pphm Calfax 16 L-35 resulted in conversion >99%, with particle size=133.7 nm and good reactor cleanliness.
Possible foaming during pre-emulsion mixing of systems with high IDMA content was a concern at higher agitation speeds. However, while we observed slightly more foam immediately after the pre-emulsion mixing stage at higher agitation, the foam quickly collapsed at the end of the three-hour feed, even at 600 rpm.
Homopolymerization of IDMA
Results of the homopolymerization of IDMA at different pre-emulsion agitation speeds are shown in Table 4.
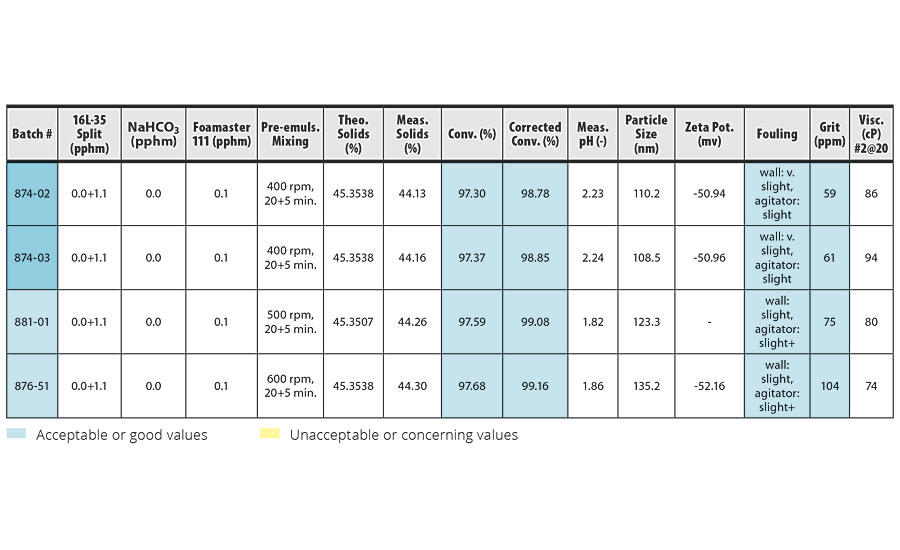
TABLE 4 » Effect of pre-emulsion agitation in IDMA homopolymerization.
In these experiments, no buffer and 0.1 pphm defoamer was used. For easier comparison with the results of our previous work,1,2 we also assumed the activity of IDMA = 100% for IDMA homopolymerization in this work. At higher agitation speeds (500, 600 rpm), we observed higher foam right after the pre-emulsion mixing. However, by the end of the 3-hr pre-emulsion feed, most of the foam had collapsed. By 5-10 min before the end of the feed only a thin layer of foam was observed. We were able to manage the feed with small amount of foam at the end by only 2-4 min longer without increasing the pump speed.
Similar to the emulsion polymerization of VeoVa 10/IDMA = 50/50, Table 4 shows the higher agitation speed resulted in higher conversion and larger particle size. However, the increase in conversion due to higher pre-emulsion agitation was smaller than in the IDMA/VeoVa 10 = 50/50 trials.
Agitation speeds over 400 rpm resulted in slightly reduced reactor cleanliness. This slight additional fouling may be due to small amounts of foam entering the reactor after the pre-emulsion mixing phase, but no foam was visually observed.
Homopolymerization of TBMA
Results of the homopolymerization of TBMA at different pre-emulsion agitation speeds are shown in Table 5.
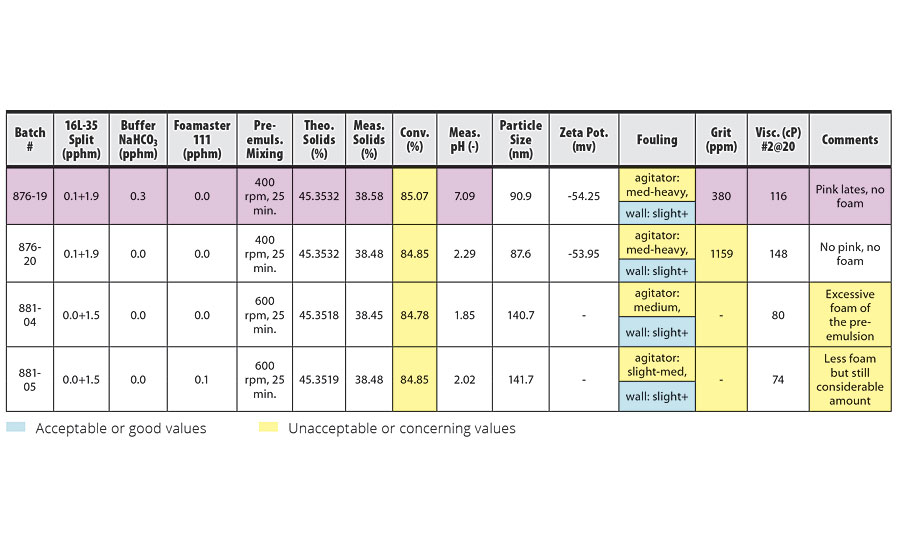
TABLE 5 » Effect of pre-emulsion agitation in TBMA homopolymerization.
The emulsion homopolymerization of TBMA, as shown in Table 5, resulted in very poor conversion and reactor fouling. Based on the results of the IDMA homopolymerization and IDMA/VeoVa 10 = 50/50 copolymerization, we expected to see an increase in conversion at 600 rpm pre-emulsion mixing, even with larger particle sizes. However, the results showed no change in conversion. Although the effect of higher pre-emulsion agitation might be complicated by the excessive foam in this system, the conversion rate of approximately 85% was far below the target of 97%.
The use of buffer NaHCO3 in this system resulted in a pink latex. Reducing the level of surfactant from 0.1+1.9 pphm to 0.0+1.5 pphm in order to increase particle size from 88 nm to 141 nm resulted in less reactor fouling.
Emulsion Polymerization of VeoVa 10 with Maleic Acid
The results of the IDMA/VeoVa 10 copolymerization in Tables 2 and 3 showed that incorporation of acid monomers produced negative effects on both polymerization conversion and reactor cleanliness. Higher levels of acid monomer and the use of more water-soluble acid monomers (such as AA vs. MAA) increased these negative effects.
In order to magnify these effects, we incorporated maleic MA, an even more hydrophilic acid monomer, into an emulsion polymerization system with VeoVa 10. Pre-emulsion agitation was held constant at 500 rpm for 25 min in these experiments and the level of Calfax 16 L-35 was held constant at 0.1+1.9 pphm. The results of VeoVa 10/MA experiments are shown in Table 6.
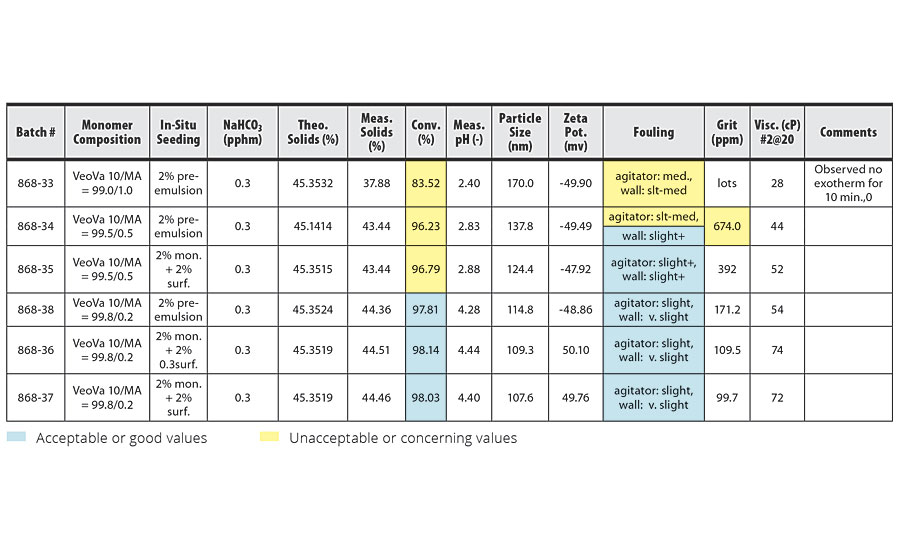
TABLE 6 » Emulsion polymerization of VeoVa 10 monomer + MA (pre-emulsion 500 rpm for 25 min).
The incorporation of MA at 1.0 pphm (the highest level used) led to significantly worse conversion and reactor cleanliness than the incorporation of 2.0 pphm AA (Batch 876-26 in Table 2). The incorporation of MA also magnified the effects of enlarging the latex particle size. The highest level of MA we were able to use with VeoVa 10 while still maintaining acceptable conversion rates and reactor cleanliness was 0.2 pphm.
To minimize these negative effects from the incorporation of acid monomers, we ran several batches that eliminated the acid monomers from the seeding stage of the emulsion polymerization. In batches 868-35, 868-36 and 868-37 (Table 6), we seeded the emulsion polymerization with 2% of the main monomer and 2% of the surfactant rather than 2% of the pre-emulsion mixture. No acid monomer was introduced into the system until after the seeding stage, when the pre-emulsion mixture was added to the reactor. The results from these batches show improved conversion and reactor cleanliness over comparable batches that used 2% of the pre-emulsion mixture during the seeding stage.
Thermal Properties of Copolymers of VeoVa 10 and/or IDMA
DSC can be used to measure a number of characteristic properties of a sample, including fusion and crystallization events as well as the glass transition temperature of a polymer (Tg). Glass transition temperature is the temperature where the polymer transitions from a hard, glassy material to a soft, rubbery material. The glass transition appears as a step in the baseline of the recorded DSC signal. This is due to the sample undergoing a change in heat capacity; no formal phase change occurs.4
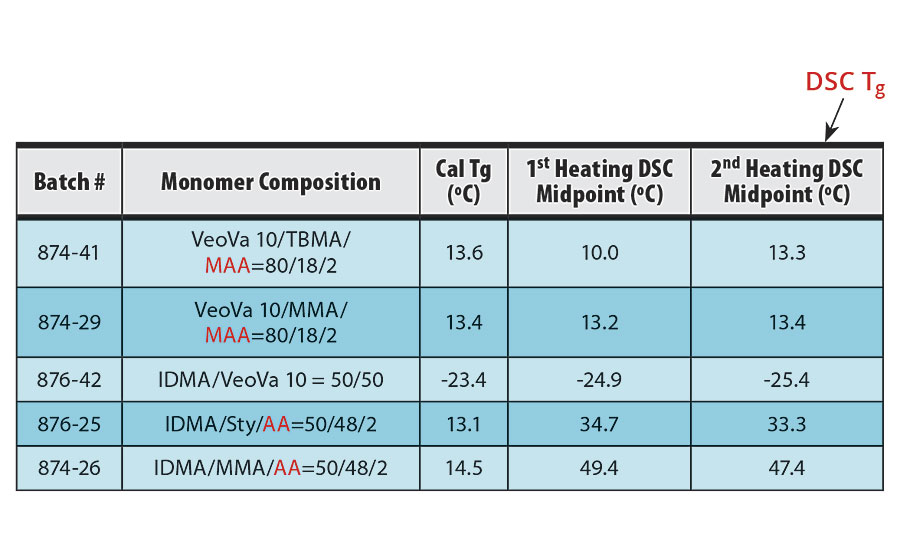
TABLE 7 » DSC data of the copolymers of VeoVa 10 and/or IDMA.
The midpoints of the DSC scans from the first heating and second heating of each sample are summarized in Table 7 along with the calculated glass transition temperatures of the copolymers. Because noise and artifacts are often seen in the first heating scan, the midpoint of the second heating transition is conventionally taken as the glass transition temperature.4,5 The second heating plots are seen in Figures 1-5.

FIGURE 1 » DSC plot of 874-41 – VeoVa 10/TBMA/MAA = 80/18/2.
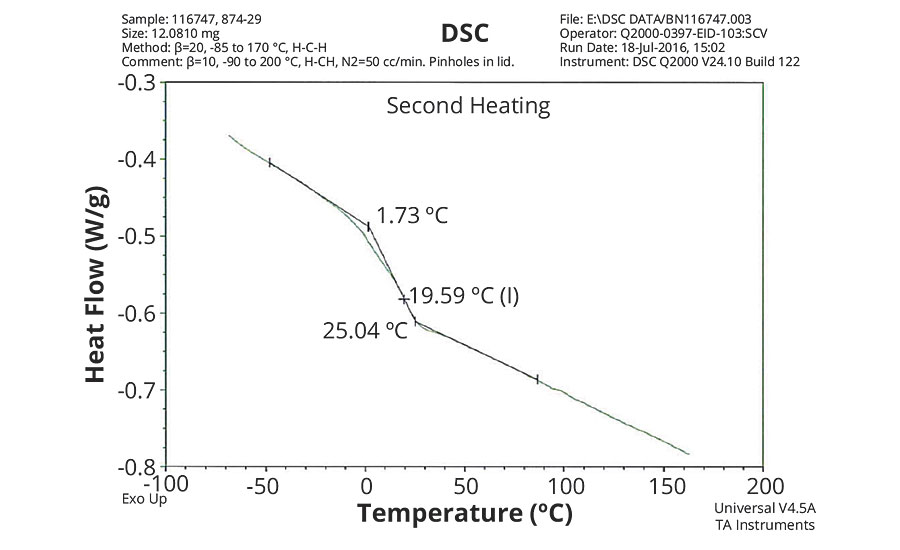
FIGURE 2 » DSC plot of 874-29 – VeoVa 10/MMA/MAA = 80/18/2.
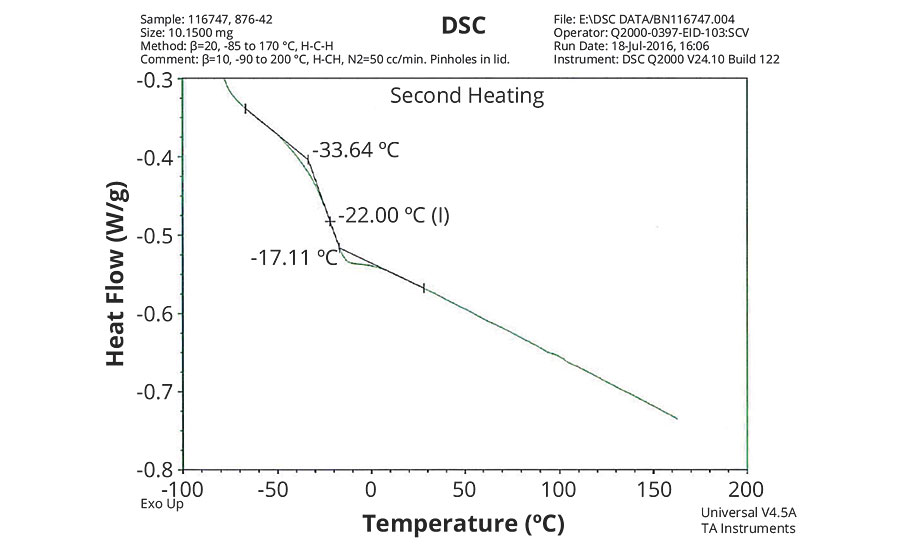
FIGURE 3 » DSC plot of 876-42 – IDMA/VeoVa 10 = 50/50.
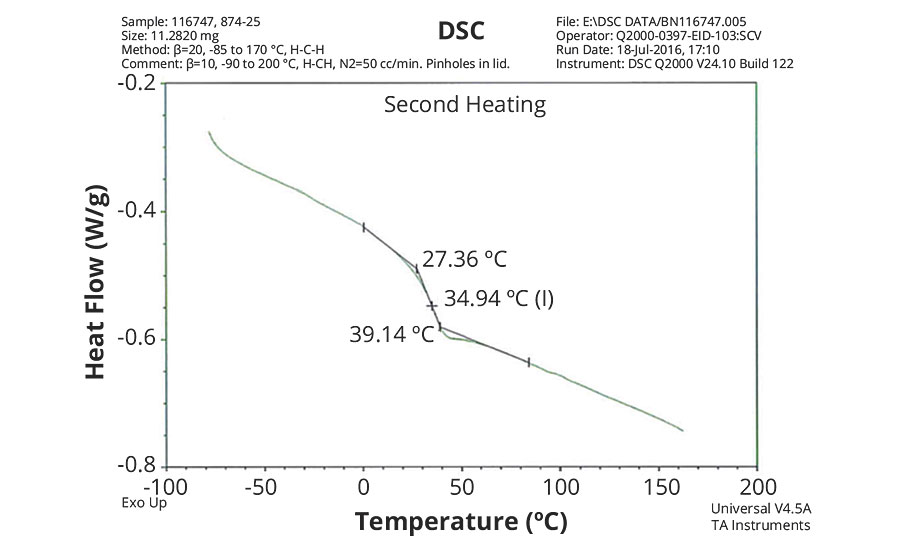
FIGURE 4 » DSC plot of 874-25 s– IDMA/Sty/ AA = 50/48/2.
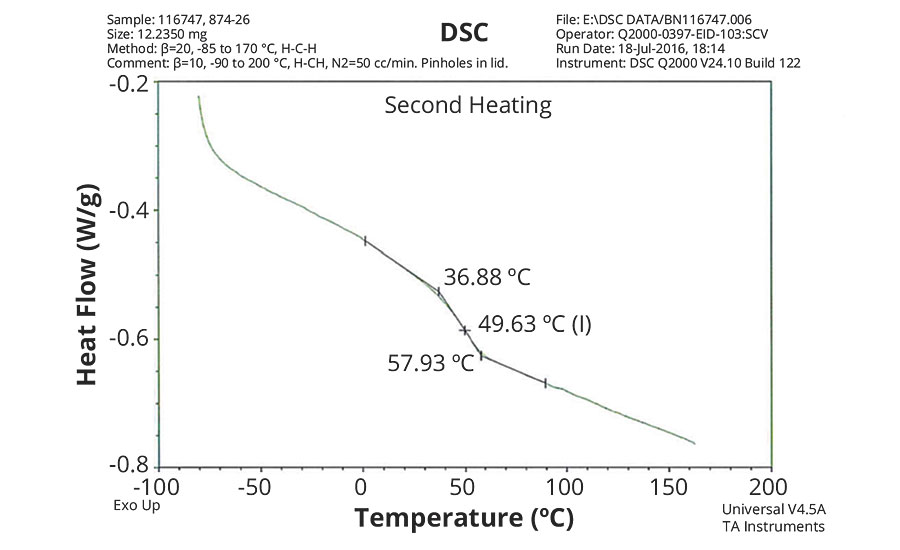
FIGURE 5 » DSC plot of 874-26 – IDMA/MAA/AA = 50/48/2.
Discussion
Effects of Pre-Emulsion Agitation
In these experiments we are dealing with systems that have a very hydrophobic composition compared to current emulsion polymer systems. Conversion rates for such systems, without a chase process, are generally in the range of 98.0-99.0%.
Conversion
Based on the results shown in Tables 1-3, pre-emulsion mixing at higher agitation significantly increased the conversion of copolymerization of IDMA and VeoVa 10 monomer, with or without acid monomer. This effect likely occurs because a higher degree of agitation during pre-emulsion mixing allows the monomer to more completely associate with the surfactant, ensuring effective monomer transport during the polymerization.
While higher surfactant levels resulted in smaller particle size and consequently higher conversion for the same monomer composition, the results clearly showed that the effect of particle size on conversion was weak compared to the effect of pre-emulsion agitation. These effects are more clearly illustrated in Figures 6 and 7, which plot conversion against agitation speed for the experiments with 1.55 and 0.8 pphm Calfax 16L-35.
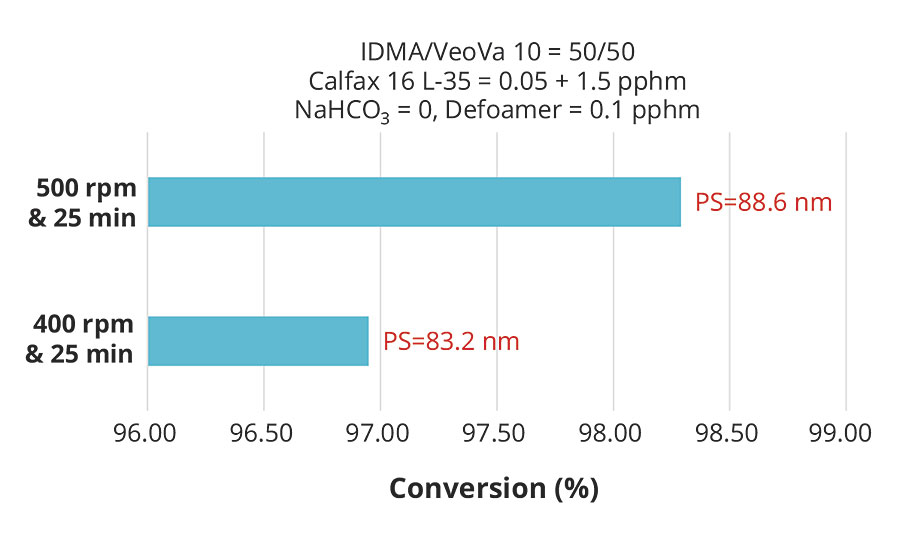
FIGURE 6 » Effect of pre-emulsion agitation on conversion with 1.5 pphm Calfax 16L-35.
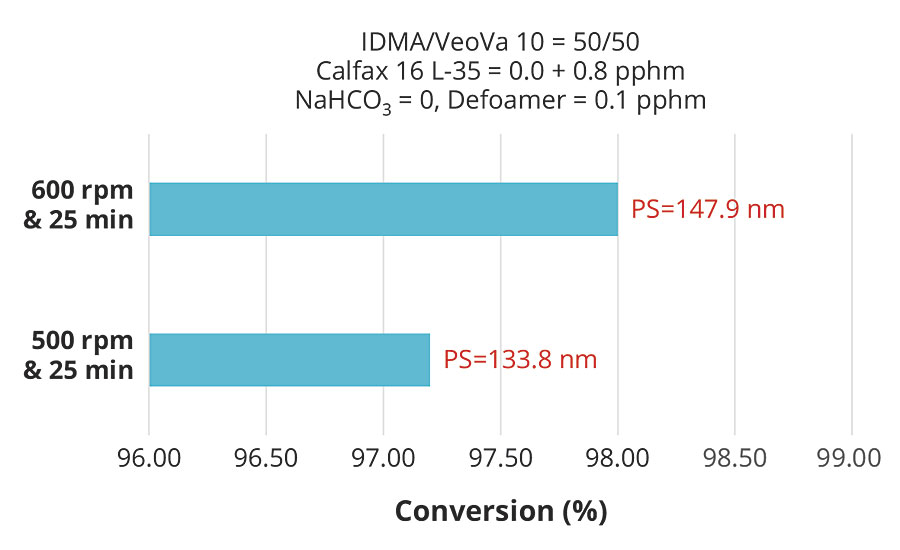
FIGURE 7 » Effect of pre-emulsion agitation on conversion with 0.8 pphm of Calfax 16 L-35.
Particle Size
We found that higher pre-emulsion agitation increased latex particle size. This increase in latex particle size might be explained on the basis that the more complete distribution of surfactant into monomer droplets reduces the number of surfactant micelles present during the particle formation phase.
While the effects of particle size on the reaction rate and the conversion of the emulsion polymerization are well known, the results here showed that these effects were overridden by the effects of having higher monomer concentration in the particles due to higher pre-emulsion agitation. The net effect of pre-emulsion agitation on latex conversion was therefore very significant, and we emphasize that pre-emulsion agitation is the most important factor in the emulsion polymerization of hydrophobic monomers.
Reactor Cleanliness
For IDMA/VeoVa 10 copolymerization batches with a pre-emulsion mixing time of 25 min, increasing mixing speed from 400 rpm to 500 rpm improved the reactor cleanliness. However, no significant further improvement in reactor cleanliness was observed when increasing agitation speed from 500 rpm to 600 rpm.
At particle sizes of 100 nm or smaller, poor reactor fouling was observed, likely because smaller distance between hydrophobic particles increase the chance for the particles to collide and coalesce under shear pressure during the polymerization.
One of the concerns we had about emulsion polymerization with a high content of IDMA was possible foaming due to higher agitation in the pre-emulsion mixing. We did observe slightly more foam at higher agitation right after the mixing; however, under the experimental conditions the foam was easily broken at the end of the 3-hr feed, even at 600 rpm, and no adverse effect was observed.
Effects of Acid Monomers
The presence of polymerized acid in a polymer is often undesirable for coatings and moisture-sensitive applications such as corrosion control, as it increases the affinity of the polymer for water. However, in certain applications, acid monomers are used to improve latex stability and adhesion properties of the polymer.
The effects of acid monomers in the copolymerization of VeoVa 10 and IDMA were studied in the experiments at 400 rpm and 500 rpm, as shown in Tables 1 and 2. In trials with a similar monomer composition and equal surfactant levels, we observed a reduction in conversion when incorporating acid monomer, with greater reductions from higher levels of acid monomer. The incorporation of AA led to a greater reduction than the incorporation of MA.
In addition, the incorporation of acid monomer led to an increase in latex particle size, which might partly account for the reduction in conversion. Similar negative effects on reactor cleanliness from the presence of acid monomers were also observed.
Taken together, these effects suggest that the more hydrophilic the acid monomer, the more difficult it is to incorporate it with very hydrophobic main monomers.
TBMA Homopolymerization
TBMA is a C4 methacrylate and is therefore not as hydrophobic as VeoVa 10 or IDMA; however, it is likely to offer good weathering properties due to its tertiary structure.
Unlike in the IDMA/VeoVa 10 copolymerization or the IDMA homopolymerization, increased agitation speed did not produce any change in conversion rate during TBMA homopolymerization. The effects of higher agitation during TBMA homopolymerization may have been offset by the excessive foam observed in this system.
The low conversion rate during TBMA homopolymerization may be attributed to the low reactivity of this monomer. In order to achieve an acceptable conversion rate, it may be necessary to co- or terpolymerize limited quantities of TBMA with other monomers.
Incorporation of Acid Monomers
As discussed above, the incorporation of acid monomer into the emulsion polymerization of very hydrophobic monomers reduces conversion and reactor cleanliness. To explain these negative effects, let’s consider what might be happening during the emulsion polymerization of a hydrophobic monomer in the presence of an acid monomer.
During the seeding stage, the acid monomer in these systems stays in the water phase, while only a very small amount (<0.001%) of the hydrophobic main monomer is present in the water phase. We theorize that since the initiator radicals form in the water phase, these are much more likely to form oligomers with the hydrophilic acid monomer than the hydrophobic main monomer, and the acid monomer molecules are more likely to homopolymerize with each other than to interact with the hydrophobic main monomer. The greater the difference between the hydrophobicity of the acid monomer and the main monomer, the more pronounced these effects are likely to be.
The result is a reduced number of “z-mer” radicals. Since only these z-mer radicals are capable of entering the micelles to initiate the micellar emulsion polymerization, the reaction rate and therefore the conversion of the emulsion polymerization are reduced as the number of z-mer radicals is reduced. In addition, some of the macromolecular materials might precipitate in the water phase or bridge with the polymer particles, resulting in reactor fouling.
In order to minimize these effects, we tested the elimination of the acid monomer from the seeding stage where the polymer particles are formed. The results shown in Table 6 suggest that seeding the emulsion polymerization system with 2% of the main monomer and 2% of the surfactant rather than the standard 2% of the pre-emulsion mixture mitigates the negative effects of incorporating acid monomers into a highly hydrophobic emulsion polymerization system.
We theorize that this improvement occurs because allowing polymer particles to form in the seeding stage before the acid monomer is added permits improved particle formation and capture of z-mer radicals once the full pre-emulsion mixture is added, at least during the initial stages of the reaction.
During these experiments, the initial charge of monomer and surfactant used in the seeding stage was mixed in water at 150 rpm, the usual agitation speed for the initial polymerization stages. We expect that further improvements in conversion and reactor cleanliness could be obtained by mixing a part of the initial reactor charge (without acid monomer) at higher agitation speeds before the start of the polymerization. In future work it may also be worthwhile to explore an external seed process; for VeoVa 10 polymerizations, a vinyl acetate seed or an acrylic seed would likely be appropriate.
Thermal Properties of Copolymers of VeoVa 10 and/or IDMA
The DSC results shown in Table 7 and Figures 1-5 show that the method outlined in this article for copolymerization of VeoVa 10 monomer or IDMA with other monomers or with each other resulted in random copolymers with good incorporation of the monomers into the resulting copolymer.
While the measured Tg for the copolymers of VeoVa 10 monomer with other monomers including VeoVa 10/IDMA copolymer were consistent with calculated values, Tg for the IDMA copolymers with styrene and MMA shown in Figures 4 and 5 were significantly higher than expected. The incorporation of MAA into the polymer has a greater effect on the transition point than the incorporation of styrene. Molecular weight and other polymer characterizations might be required to better understand the higher transition points of IDMA copolymers.
Conclusions
This work demonstrated that through the use of a single surfactant, Calfax 16 L-35, a simple emulsion polymerization process can generate 45% hydrophobic latex with particle size in the range of 100-150 nm at a conversion rate of >98% at the end of a 4-hr reaction, without a chase process. DSC measurements indicated that this process resulted in random copolymers of VeoVa 10 and IDMA with each other and with other monomers.
The agitation speed used during pre-emulsion mixing was found to be critical to both conversion and reactor cleanliness for the emulsion polymerization of hydrophobic monomers. This was particularly true for very hydrophobic systems, such as the copolymerization of VeoVa 10 and IDMA or the homopolymerization of either of these monomers. With mixing time = 25 min, we found 600 rpm mixing resulted in significant improvement over 400 or 500 rpm. Future work to explore even higher pre-emulsion agitation speeds and longer mixing times may yield further benefits.
The incorporation of acid monomers was found to reduce conversion and reactor cleanliness for more hydrophobic systems; however, these negative impacts could be partially offset by removing the acid monomer from the seeding stage. The copolymerization or terpolymerization of IDMA or VeoVa 10 with less hydrophobic monomers such as styrene and MMA was not significantlty affected by the presence of acid monomers.
While we have addressed the effect of some polymerization parameters in this work, better understanding of the reaction mechanism as well as the characteristics and benefits of the hydrophobic polymer systems will require additional efforts across academia and industry. n
References
1 Nguyen, B.D. 43rd Annual International Waterborne, High-Solids, and Powder Coatings Symposium, Additives session.
2 Nguyen, B.D. Paint & Coatings Industry Magazine, June 2016, page 54-62.
3 Certificate of Analysis of Bisomer IDMA, Material number: 678508, Batch No.: 2042927111.
4 Differential scanning calorimetry, from Wikipedia, the free encyclopedia.
5 Jin, J.M.; Lee, J.M.; Ha, M.H.; Lee, K.; Choe, S. Polymer, vol.48, issue 11 (21 May 2007), page 3107-3115.
6 European Coatings Forum – Testing & Measuring/Tg measurement /September 13, 2005.
Acknowledgements
I would like to express my special thanks to Mr. Rick F. Shook and Mr. Ryan J. Connair at the Pilot Chemical Company for their great help in proof reading and suggestions in the writing of this article.
VeoVa™ is a trademark of Hexion, Inc.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!