The Science of Smart Hydrophobic Coatings



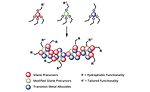
FIGURE 1 » Hydrophobically modified mixed metal oxides.

FIGURE 2 » Surface energy results of Powdura Polyester TGIC powder coating with varying amounts of cationic silica additive.

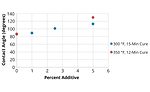
FIGURE 3 » Water contact angle measurements of Powdura Polyester TGIC powder coating with varying amounts of cationic silica additive.

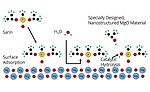
FIGURE 4 » Adsorption of sarin model compound onto magnesium oxide (Ref: Morris Group Virginia Tech).

TABLE 1 » Coating performance.




Hydrophobic (water-repelling) coatings have numerous applications, from protecting critical infrastructure to developing stain-repellant textiles. New advances in material science are making it possible to design smart, functional coatings that combine hydrophobic properties with added functionality for specific industrial, consumer, medical or military applications. Here’s a look at how these smart coatings are made and how they may be used in the future.
Creating Smart, Functional Coatings
What do we mean by a “smart” or functional coating? Simply put, it’s a coating that has been engineered to react to its environment in specific ways. Functional coatings can be created to respond to specific stimuli such as the presence of water or other chemicals, or physical stimuli such as temperature, electricity or light.
Hydrophobic materials and coatings have been used for decades. Now, material scientists are going beyond simple water repellency to incorporate additional functionality for more specific applications. Functional coatings are created by manipulating the nanostructure or chemical composition of the coating itself or introducing additives in order to change the way the coating responds in the presence of specific stimuli. These stimuli-responsive materials are able to chemically or physically react to their environment in various ways. For example:
- Self-cleaning coatings can react with specific contaminants in order to break them down into less problematic byproducts.
- Responsive polymer coatings have been created that can change their surface topography in response to light in order to modulate characteristics such as wettability or surface friction.1
- The Battelle Smart Corrosion Detector® capsule detects chemical signatures of corrosion and releases a healing agent to repair the damage.
The Creation of a Self-Decontaminating Powder Coating Additive
One example of a smart hydrophobic surface is a new chemical agent-resistant coating (CARC) designed by Battelle for the U.S. military. CARCs are used to protect military infrastructure, vehicles and equipment from chemical agents such as mustard gas and nerve agents (saran or VX) in combat zones. Traditionally, CARCs are made by adding high loads of the same pigments and extenders that are used in commercially available paints. This high loading makes the paint somewhat hydrophobic, slowing the absorption of chemical agents into the coating and buying time for decontamination.
By contrast, the powder coating developed at Battelle both actively repels and decontaminates chemical agents for more effective and longer-lasting protection. Instead of just shedding of the chemical agent, the coating actually breaks down remaining traces of the contaminant into less harmful substances. The new CARC has the potential to significantly reduce cleaning and paint costs for the military. Rather than going through expensive decontamination or demilitarizing assets, field personnel can rely on the paint to decontaminate itself. The material can be added to any paint or coating in the form of a powder.
The smart CARC coating has dual functionality (Figure 1) that allows it to both repel water-based chemicals and decontaminate itself:
- A modified mixed metal oxide provides the hydrophobic properties.
- A cationic silica group was engineered to decontaminate the surface by reacting with any remaining traces of chemical agent and breaking it down into harmless compounds.
In testing, the cationic silica additive was combined with Powdura Polyester TGIC® powder at varying amounts (0-5 w/w%) and applied to Prekote® treated steel and aluminum panels. After applying and curing the coatings, the chemical resistance of the coatings was tested with surface wettability, surface energy (Figure 2), MEK rub and roll off tests (Figure 3). These tests demonstrated that the cationic silica additive provides superior resistance to chemical agents, with resistance increasing as increased levels of the additive are used. In addition to these tests of chemical resistance, tests of mechanical properties were performed as well (Table 1), which demonstrated that coatings with the additive maintained good mechanical properties. Compared to traditional powder coatings, the functional cationic silica coating demonstrated higher water resistance, lower surface energy, greater solvent resistance and an increase in chemical agent resistance.
The ability to decontaminate was also designed into this CARC coating. Metal oxide powders such as magnesium oxide, alumina, zinc oxide, calcium oxide and titania have been studied for decontaminating chemical agents.2-7 It was suggested that the chemical agents adsorbed onto the metal oxides (Figure 4), and oxidation and hydrolysis of the chemical agents occurs at the adsorbed sites. This results in the conversion of chemical agents to nonhazardous (or less hazardous) products.
The functional silica group can be customized to meet different performance requirements. Similar surface decontamination technology could be engineered for other applications in the consumer and industrial space, such as industrial paints that react with toxins and carcinogens to break them down into less harmful substances. Surface decontamination could also be used in fabrics and equipment used by emergency responders and factory or lab workers who may be exposed to toxic or carcinogenic substances.
Additional Applications for Smart Hydrophobic Coatings
The applications for smart hydrophobic surfaces go far beyond decontamination of chemical agents and toxins. Functional coatings can be designed for a wide variety of purposes, such as:
- Antimicrobial: Antimicrobial coatings could help hospitals cut down on cross-contamination of surfaces and hospital-acquired infections. Antimicrobial coatings use hydrophobic properties to prevent adhesion from microorganisms. Additional functionality can be added through functional groups that are able to recognize chemical signatures of bacteria and take specific actions, such as turning a different color or altering electrical properties to indicate contamination.
- Anti-Biofouling: Offshore infrastructure and sea-going vessels are prone to biofouling by small marine organisms such as mussels and barnacles. Functional coatings can be designed to reduce the ability of marine organisms and bacteria to adhere to ships, oilrigs and mooring chains, and other offshore infrastructure. Reducing biofouling in the shipping industry not only protects the vessel and reduces drag, but could also help to reduce the spread of invasive species across the globe.
- Anticorrosion: Functional coatings can provide better protection for utility infrastructure, oil and gas pipelines and equipment, consumer and military vehicles, and other infrastructure components. In addition to the Smart Corrosion Detector capsule approach described earlier, functional surfaces could be designed to detect and respond to the presence of corrosion-causing bacteria.
- Anti-Icing: Hydrophobic surfaces can reduce ice buildup on airplane wings, utility infrastructure and other outdoor components.
Developing a coating that meets all the requirements of a coating while providing one of these additional functions is a challenging and complex task. The one thing we can be sure of is that 10 years from now, we will be looking to develop coatings that provide additional functions even more complex than those listed previously.
Design Considerations for Functional Coatings
Designing a functional hydrophobic surface requires careful balancing of functional requirements against other mechanical and performance specifications along with economic and regulatory factors. Changing the coating formulation to introduce functional groups may change its properties in other ways that aren’t entirely predictable; the resulting surface may be softer, less durable, less colorfast or demonstrate other negative impacts on coating performance. Alternatively, the formulation may depend on materials that are expensive, hard to source, hazardous or toxic, or subject to burdensome regulation. Finding the right solution requires material scientists to evaluate a number of factors:
- Desired Functionality and Performance: What does the coating need to do? What are the performance specifications that it needs to meet (e.g., hardness, durability, etc.)? What is the intended effective lifetime?
- Use Environment: Where will it be used, and by whom (e.g., hospital, household or military field conditions)? What will the material be exposed to during use (e.g., water, chemicals, or other materials)? What other materials must it be compatible with, including the substrate and other materials it may come in contact with?
- Supply Chain, Manufacturability and Economic Issues: Is the proposed solution economically viable? Can it be easily scaled up for production? Does it rely on rare materials with limited suppliers? How expensive are the materials? What price point does the final product need to stay within?
- Safety, Environmental and Regulatory Issues: What types of regulation is the product subject to? Will it need to pass review by the U.S. Food and Drug Administration or other regulatory body? What is the environmental impact across the lifecycle, including manufacturing, use and disposal (e.g., does the coating shed chemicals or nanoparticles)?
Material scientists must look at all of these factors when designing a smart hydrophobic coating; a functional coating that meets performance specifications but cannot be scaled up for production or does not pass regulatory review will not be able to be commercialized. At Battelle, scientists in the Surface Science program are working to design market-ready coatings that meet specific functional and performance requirements for military, industrial and consumer applications.
As we continue to make advances in material science, particularly in nanomaterials and stimuli-responsive materials, the possibilities for smart hydrophobic coatings are nearly limitless. Tomorrow’s paints and coatings can be much more than a pretty surface. Many of them will be smart, functional materials that respond to - and change - the environments around them.
By Robert Cain, Rachel Krebs, Ram Lalgudi, Ph.D., and Steven Risser, Ph.D., Battelle, Columbus, OH
References
1 Stumpel, J.; Broer, D.; Schenning, A. Stimuli-Responsive Phototonic Polymer Coatings. Chemical Communications. Issue 100, (2014, September). Downloaded November 2016 from http://pubs.rsc.org/en/Content/ArticleLanding/2014/CC/C4CC05072J#!divAbstract.
2 Decontamination of Chemical Warfare Agents with Nanosize Metal Oxides. In Defense Applications of Nanomaterials, American Chemical Society: Washington, DC, 2009; p. 139-152.
3 George, W.W. Decontamination of Chemical Warfare Agents with Nanosize Metal Oxides. In Nanoscale Materials in Chemistry: Environmental Applications, American Chemical Society: 2010; Vol. 1045, p. 125-136.
4 Mahato, T.H.; Prasad, G.K.; Singh, B.; Acharya, J.; Srivastava, A.R.; Vijayaraghavan, R. Nanocrystalline Zinc Oxide for the Decontamination of Sarin. Journal of Hazardous Materials 2009, 165 (1–3), 928-932.
5 Martyanov, I.N.; Klabunde, K.J. Photocatalytic Oxidation of Gaseous 2-Chloroethyl Ethyl Sulfide over TiO2. Environmental Science & Technology 2003, 37 (15), 3448-3453.
6 Prasad, G.K.; Mahato, T.H.; Singh, B.; Pandey, P.; Rao, A.N.; Ganesan, K.; Vijayraghavan, R. Decontamination of Sulfur Mustard on Manganese Oxide Nanostructures. AIChE Journal 2007, 53 (6), 1562-1567.
7 Sheinker, V.N.; Mitchell, M.B. Quantitative Study of the Decomposition of Dimethyl Methylphosphonate (DMMP) on Metal Oxides at Room Temperature and Above. Chemistry of Materials 2002, 14 (3), 1257-1268.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!















