Spherical Precipitated Silica
Next-Generation Particle Morphology for Performance in Coatings

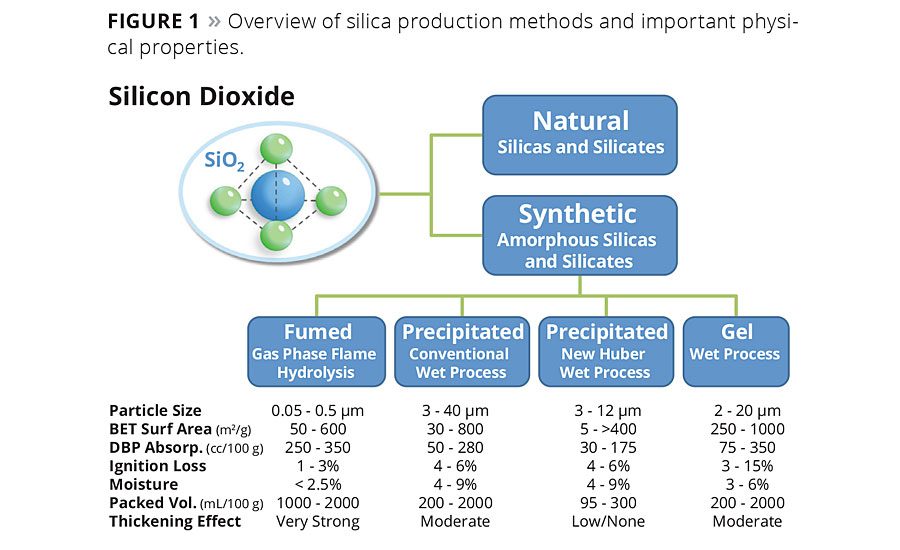
Figure 1
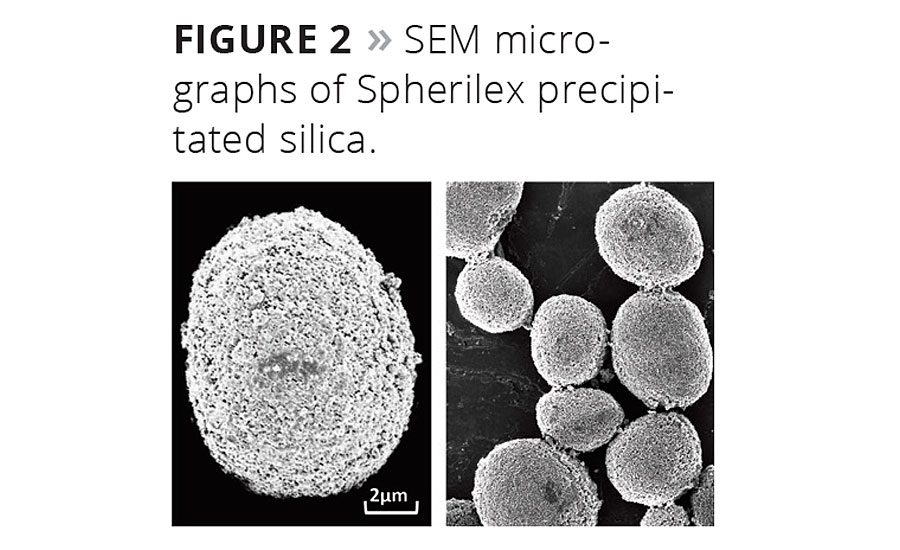
Figure 2

Figure 3

Figure 4
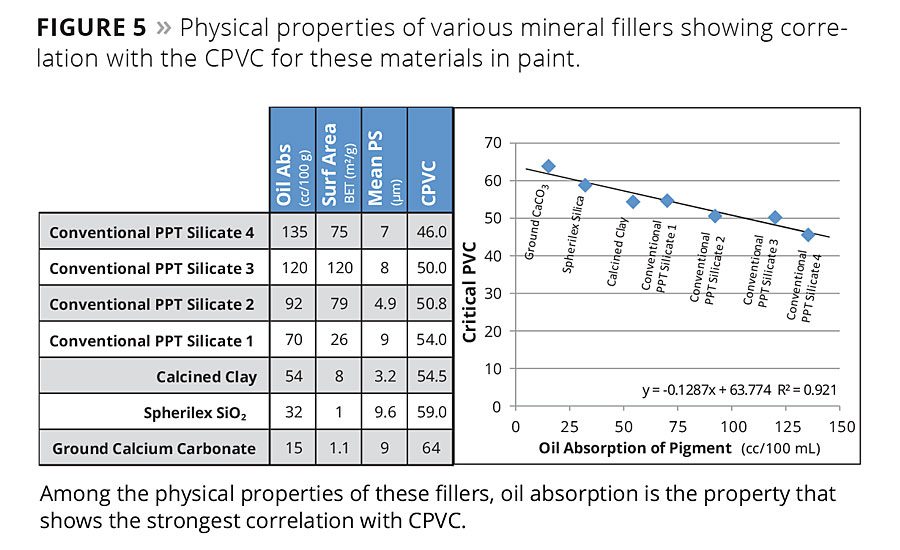
Figure 5
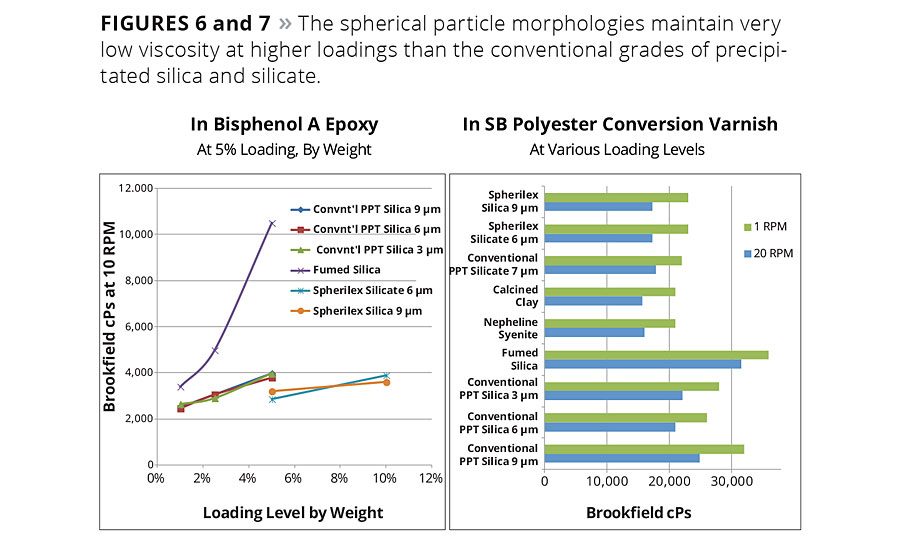
Figure 6 and 7
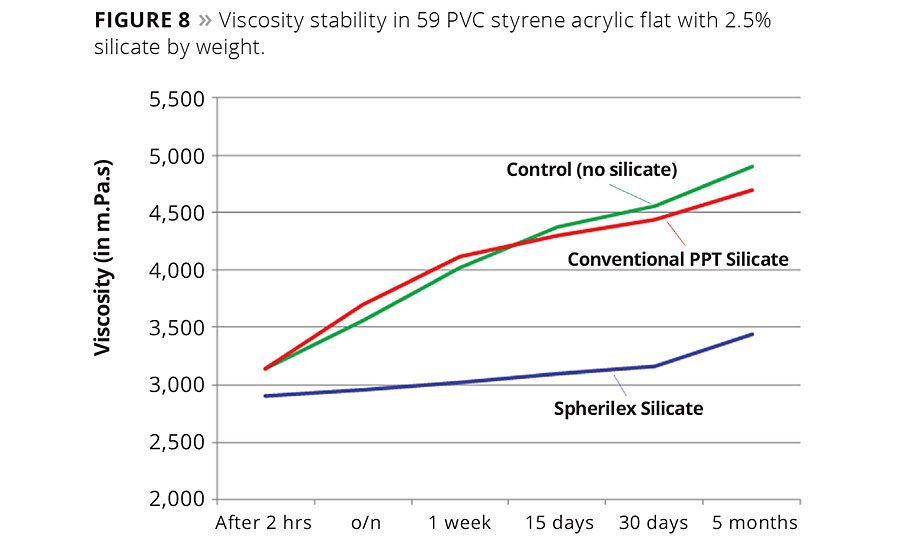
Figure 8
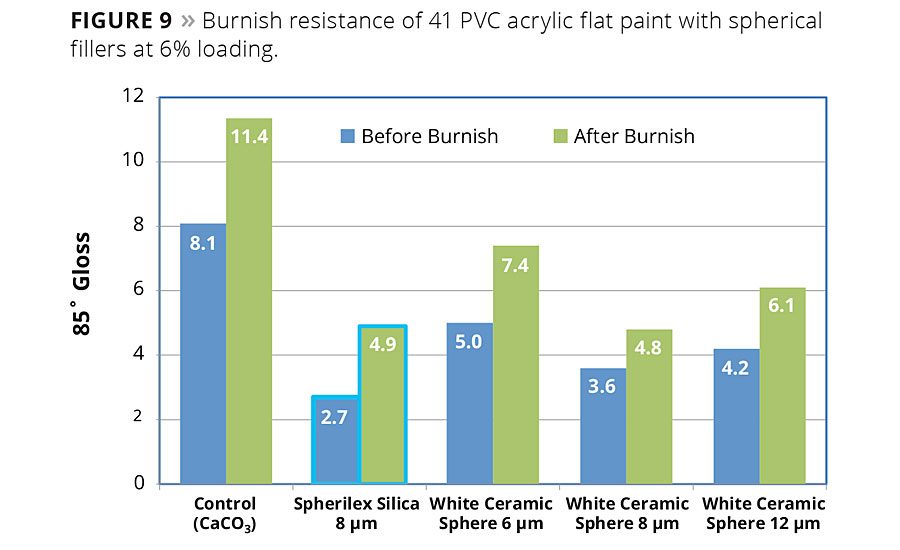
Figure 9
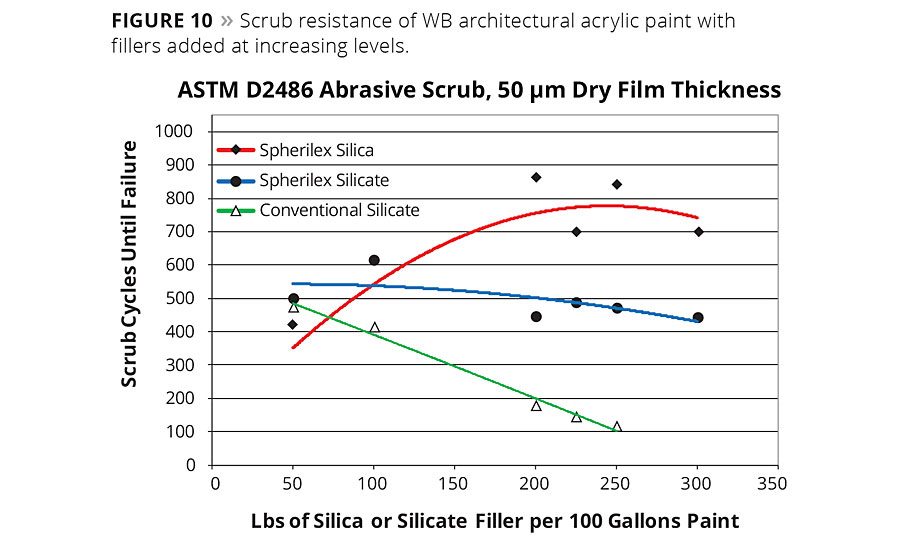
Figure 10

Figure 11

Figure 12

Table 1











Silicon is the eighth most abundant element in the universe. Silicon dioxide (or silica) is present in over 90% of the minerals that make up the earth’s crust. Man’s use of silica-based minerals dates back to the very origins of recorded human history. Ancient man used flint to strike sparks for the fires they needed to stay warm, while today modern man can be protected from heat of reentry into the Earth’s atmosphere thanks to silica-based tiles that protect the space shuttle.
In paints and coatings, siliceous minerals were known to have been used as pigments in the oldest surviving cave paintings dating back to 40,000 BC. Naturally occurring forms of silica and silicate are still used today as functional fillers, with the silicon atoms determining much of the overall character of these minerals. Silica forms tetrahedral arrangements. The tendency of these units to form a three-dimensional framework is fundamental to silica crystal chemistry, which in turn directs the arrangement of the minerals’ structure into the lattice shapes that determine their particle shape and other characteristics.1,2
Natural vs. Synthetic Silica
Naturally occurring siliceous mineral fillers include silicon dioxide in forms such as quartz and cristobalite, or silicate like mica, talc and kaolin. The naturally occurring minerals typically contain some amount of crystalline silica, either as a minor component or as the principal component of their makeup. While many of the characteristics of the natural materials are ideal as fillers for coatings (low binder demand, inertness, etc.), crystalline silica has gained much recent attention based on health concerns and regulatory developments. (It is important to note that synthetically produced materials are amorphous and typically do not contain crystalline phases of silica.)
Jöns Jakob Berzelius is credited as being first to isolate silicon and characterize it as an element in 1824, which paved the way for a considerable history of advances in silicon-based chemistry.3 The first synthetic silicas began commercial production around the time of World War I. The synthetic routes produced materials with higher purity than the natural mineral varieties, and enabled chemical and morphological customizations that expanded the utility of silicas into new applications. Today’s synthetic silicas are highly specialized materials that have become critical ingredients in toothpaste, rubber tires, plastics and coatings, with a combined global production estimated at 3.2 million metric tons for 2016.4
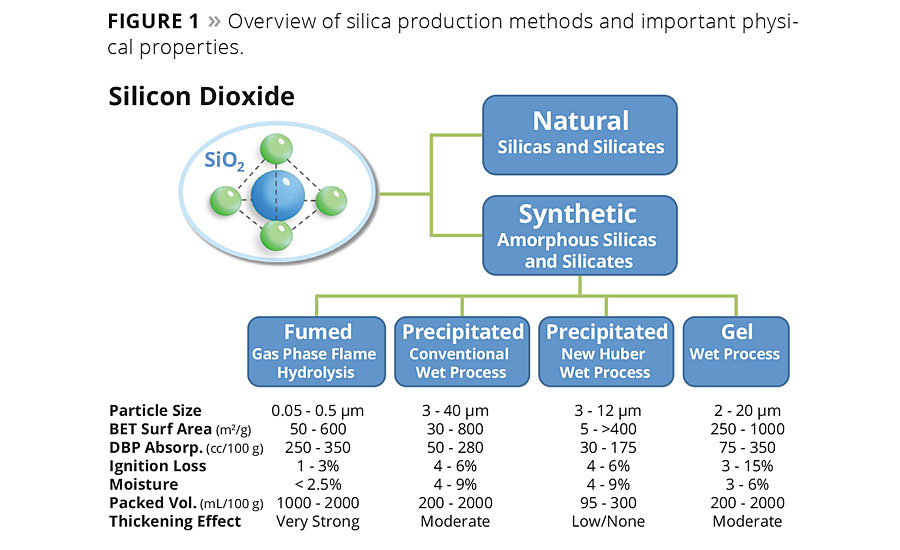
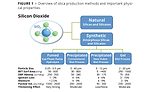
Synthetic silica is produced on an industrial scale via two primary routes that can be categorized as liquid phase or gas phase processes. Regardless of the method of preparation, all synthetic silica is amorphous in nature,5 meaning its molecular arrangements of silicon and oxygen are not patterned into the kind of lattices or crystalline structures that are found in the naturally occurring minerals. While the gas and liquid phase manufacturing processes both produce materials with amorphous molecular structures, they yield particle morphologies and other properties that are fundamentally different (Figure 1).
Gas Phase Process: Fumed Silica
Fumed silica is made by pyrogenic flame hydrolysis, which is the predominant gas phase process used today. The anhydrous nature of the pyrogenic process leaves most surface silanols condensed to siloxane bridges (-Si-O-Si-), so that silanol surface density is only about 25% of that of hydrated silicas. There is consequently less tendency to absorb water, and the products typically contain less than 2% free moisture.6
Fumed silicas range in particle size from approximately 0.05 to 0.50 µm, which is extremely small as compared to the products produced by the wet process technologies. These particles are comprised of smaller subunits of only 80-100 nm, which are loosely aggregated into chain-like arrangements that give very low density and high surface area to the overall particle structure. These chemical and morphological distinctions of fumed silica have led to its widespread use for rheology and for gloss control in paints.
Wet Process: Precipitated Silica and Silica Gel
The two most significant wet phase manufacturing routes are the gel and precipitated processes, with the latter accounting for the largest volumes of product sold in the current marketplace. Precipitated silica is produced using the conventional wet chemistry process as well as the new Spherilex™ silica wet chemistry process developed by Huber Engineered Materials. The new process produces material with a unique spherical particle morphology, providing nontraditional silica benefits in coatings.
Variations in the wet reaction process conditions allow manufacturers to control many aspects of the final product, such as pH, surface area, pore volume (within the particles), pore size distribution and other structural or morphological effects. Compared with fumed silica, the hydrated process produces materials that have a higher density of silanol groups (-Si-OH) at their surface and absorb water readily. These products are typically sold with free moisture content of 5-6%.
For the gel and conventional precipitated silica processes, the particle size and distribution (PSD) are typically controlled by milling. The Spherilex technology is able to control particle size with exceptionally narrow particle distributions during the precipitation reaction itself.
Particle size is among the most important differentiators that will determine the function and performance of the product in its end use. For example, silica products for rheology control in paints are generally less than 4 µm, whereas particle size for gloss control may be from 2-20 µm, depending on the system and applied film thickness of the coating. PSD is also important in terms of dispersability, whereas the finest particles tend to form the most stable agglomerates and the top-sized particles cannot be dispersed more finely into paints without the use of high-intensity media mills.
New Technology for Old Challenges
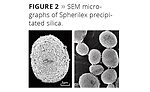
The true value of modern synthetic silica technology is that it expands the functionality of the basic silica chemistry beyond the scope of what the naturally derived minerals can do. The new technology process adds to the functionality of precipitated silica by allowing many aspects of particle morphology to be customized during the precipitation reaction itself. The new process can produce both silica and aluminosilicate materials with uniquely spherical particle morphology (Figure 2), exceptionally narrow particle distribution and a range of structural variations within the spherical particles, as shown in Table 1.
Overall, the most critical differentiators that determine the functional characteristics of synthetic silica are its particle size, size distribution and particle morphology. The new process is able to produce materials within a broad range of physical properties, but it is the combination and balance of these properties that makes the new products unique as compared to other fillers. In the context of paints and coatings, these properties include:
- Relatively inert
- Low binder demand
- Excellent scrub resistance
- Compatibility/viscosity stabilization
- Low viscosity at high solids
- Excellent burnish and abrasion resistance
- Significant matting performance (depending on particle size)
- Good transparency – for deep colors and clear coatings
- Very narrow particle distribution
- Extremely low abrasiveness.
Particle Size and Particle Size Distribution
The particle size affects many of the physical properties of a coating before, during and after the coating is applied. Rheology, gloss/sheen, touch up, toughness and abrasion resistance are just some of the properties that depend on the particle size. The largest particles tend to be more efficient for matting, while the smallest particles can be more difficult to disperse and tend to contribute more toward the rheology of the system.
Particle size distribution (PSD) is equally relevant to any properties of the coating that depend on particle size. The PSD reveals how well the mean approximates the size of the particles in the overall composition. In very broad distributions, size-dependent effects are more difficult to predict based on the mean particle size alone.
Particle size blending is a known means of achieving exceptionally low binder demand and low viscosity at high solids, particularly when working with spherical materials.7 Blending requires materials with well-controlled PSD, though most of the filler materials currently on the market are not controlled sufficiently for the full potential of this formulating strategy to have been realized by the coatings industry.8
Figure 3 shows a comparison of particle distributions of spherical silica and a commercially available example of ceramic spheres. Note these materials have significantly different particle sizes.
The entry of new narrow-distribution spherical fillers into the marketplace may therefore provide new opportunities for volatile organic content (VOC) management, higher solids systems and other innovations.
Narrow particle distributions enable formulators to target very size-specific goals in their systems. For example, narrow particle distributions demonstrate unique flow behaviors in liquid suspensions and desirable melt flows in powder coatings.9, 10, 11
Gloss and sheen optimization are primary examples of formulating goals that depend on the particle size of the pigments and fillers. To the extent that matting is a function of particle size, matting efficiency is a function of the actual number of particles available in that size range. Narrow distributions are able to concentrate a higher number of particles in a desired size range, to leverage both the size and number aspects of matting efficiency without the detrimental effects of undersized or oversized particle fractions.
The Significance of Filler Particle Morphology in Coatings
In addition to particle size and PSD, particle structure is the other major variable that manufacturers control to provide value and functionality in the silica products sold.
There are many aspects of particle shape or morphology that determine the function and performance of the filler. The shapes of synthetic silica and silicate particles can be simple or complex on many levels. The overall silhouette or outermost shape of the particle is sometimes referred to as the first-order morphology (Figure 4). The first-order morphology, which could be a plate, needle, sphere, etc., is likely to affect properties of 60° gloss, surface roughness, film reinforcement or resistance to abrasive damage.
Synthetic silica has a complex particle structure with further degrees of complexity within its overall first-order morphology. These second- and third-order attributes can significantly increase the surface area of the material as well as the binder and surfactant demand. Any porosity in the particle structure will decrease the apparent density of the particles (which is beneficial for anti-settling) and is likely to enhance matting effects.
Particle structure is one of the most important variables that synthetic silica manufacturers use to tailor the performance of their products, but the balance of structural refinements generally compromises some other aspect of performance. Highly structured particles are most efficient as matting agents, however, the same structural effects increase the binder demand and diminish the burnish and scrub resistance of the coating films.
The new silica process is able to manage the first- and second-order morphologies independently, allowing surface area and porosity within the spherical outer shape. The spherical shape allows high apparent hardness, excellent burnish and scrub resistance and very low binder demand, yet the secondary particle structure can be optimized separately for matting and other performance needs. In the scheme of siliceous filler morphology, the novel silica-based materials occupy a new design space with unique balances of properties.
Spherical Precipitated Filler Performance in Paints
The physical properties of a coating are based on the binder demand of the solids relative to the binding ability of the polymers. The relative binder demand and binding abilities of the raw materials define the critical pigment volume concentration (CPVC). At pigment concentrations above CPVC, the surrounding continuous phase of binder is no longer continuous. Air voids occur between pigment particles, and the physical integrity of the paint is dramatically reduced.
The ratio of the calculated pigment volume content (PVC) to the CPVC of the materials is a concept known as the Q-Factor7 or Lambda (l).12 Lambda estimates the overall physical integrity of the system because it reflects whether the coating is below, near or above CPVC.
The concept of Lambda has taken new significance because of the challenges with VOC reduction. Solvents are able to soften polymers, allowing them to flow more freely to surround and wet the pigment particles in a coating, thereby enhancing the binding ability of the binder. As the availability of these solvents diminishes in the marketplace due to requirements to reduce VOCs, formulators must seek new ways to minimize the binder demand of the solids in their formulations. Figure 5 provides examples of several types of fillers and demonstrates how their physical properties correlate with binder demand.
The spherical precipitated silica-based materials can have very low oil absorption values relative to conventional precipitates and many other filler types. The low oil absorption translates to low binder demand and higher performance properties in paints. Binder absorption is related to the oil absorption of the solids and their packing efficiency in the coating film.13
Spherical Precipitates and Paint Rheology
Synthetic silica tends to be very rheologically active, and viscosity control represents a major usage category for these materials. In contrast, spherical silicas and silicates have uniquely low rheology contribution, which allows these products to be used for other purposes like gloss reduction and abrasion resistance in rheologically sensitive applications where viscosity build would otherwise limit or prohibit their use.
The rheology studies in Figures 6 and 7 illustrate the relative viscosity response of spherical silica precipitates versus other types of materials and particle morphologies in two types of industrial coatings where good flow and leveling are critical. Both systems are fairly resistant to gloss reduction, so it is advantageous to maintain low viscosity at the higher loadings required to achieve the desired matting effect.
In the epoxy, the spherical silica and silicate develops significantly less viscosity than the fumed or conventionally precipitated silica. In the polyester varnish, the spherical silica shows 10-30% lower viscosity than the conventional precipitated silica.
In waterborne systems, spherical silica precipitates have demonstrated the ability to stabilize viscosity drift in architectural coatings between 40 and 60 PVC, based on acrylic and styrene acrylic lattices. In some cases, the spherical silicate was added as a partial replacement for another filler or TiO2 (Figure 8) to maintain the solids and PVC of the system. In other cases, it was simply added into the formulation as an additional component.
Abrasion Resistance of Spherical Materials
Spherical materials are known to possess abrasion resistant properties in paints, and the use of spherical fillers for burnishing resistance and scrub resistance is also well known.13 Spherilex™ silica and silicate provides many of the same benefits in coatings, yet they can be used in deep colors/clears or thin film applications where competitive technologies cannot be used due to their color or broad particle distribution.
The example shown in Figure 9 demonstrates competitive burnish performance of the spherical silica in architectural coatings versus conventional and other types of spherical fillers. The spherical silica precipitate easily surpasses the performance of the conventional precipitate and performs similarly to the ceramic spheres.
The benefits of low binder demand and spherical morphology can also be seen in different types of wet or dry abrasion tests. ASTM D2486 scrub test data (Figure 10) shows superior scrub performance of spherical silica and silicate versus conventional precipitated silicate pigment. Versus other spherical fillers (Figure 11), the spherical silica resists abrasion comparable (and better in some cases) to ceramic or glass spheres, which are based on much harder and denser materials. Moreover, it is important for the particles to remain lodged in the film in order to affect abrasion resistance and reinforcement via stress transfer within the matrix.5,14 The performance of the spherical precipitate in both wet and dry abrasive testing suggests that the second order morphological distinctions of the spherical silica may facilitate the adhesive interaction with the surrounding polymer.
Matting and Gloss Reduction
Matting studies lend important insight to the unique properties of spherical silica and silicate. Matting performance is both a function of particle size and particle morphology.
Figure 12 provides an example of a waterborne polyurethane clear finish, in which the diatomaceous silica and the precipitated Silica A (both matting agents) showed the most pronounced matting effects. Between these two, the diatomaceous product is slightly larger but has lower particle structure as reflected by the oil absorption, both of which contribute to its efficiency as a matting agent. In conclusion, particle size helped the matting efficiency of the diatomaceous material.
Based on size, it is expected the 8.6 µm ceramic spheres to perform similarly to Silica A, yet this ceramic material shows the weakest matting effect. This result speaks to the significance of particle structure in determining matting performance. Note Spherical Silica D and E showed very little matting effect as compared to Conventional Silica B. In this case again, the higher structure of the conventional precipitate provided greater matting effect.
The 11.0 µm Spherical Silica C showed some of the strongest matting performance, though it has much lower structure than Conventional Silica B (also a matting agent). Here also, the size and narrow size distribution appear to excel over the milled conventional precipitate.
In summary, it can be seen that spherical silica has the ability to selectively provide matting efficiency via particle structure or PSD effects, which do not significantly increase the binder demand, as is the case with prior generation materials.
Conclusion
Although mineral filler technologies have evolved considerably throughout the course of their use in paints and coatings, regulatory concerns and other challenges have resulted in a decreasing array of available materials from which to formulate. However, the performance requirements for coatings are increasing. In this challenging environment, true innovations will continue to be in strong demand.
The new Spherilex™ technology addresses long-standing challenges in manufacturing and also avails a new range of functional silica and silicate products with unique performance profiles that help formulators realize new value in synthetic silica technology.
For more information, contact melanie.galloway@huber.com or visit www.hubersilicas.com.
References
1 RT Vanderbilt Company. “Functional Silicate Fillers: Basic Principles.” Paint & Coatings Industry, August, 2002.
2 Bergna, H.E.; Roberts, W.O. Colloidal Silica Fundamentals and Applications. Boca Raton: CRC Press Taylor & Francis Group, 2005.
3 Ferch, H. Amorphous Synthetic Silica Products in Powder Form. Production and Characterization. Progress in Organic Coatings, volume 9 issue 2, 135-163. The Netherlands: Elsevier B.V., 1981.
4 Future Market Insights. Precipitated Silica to Account for 70% Volume Share of Global Demand for Specialty Silica in 2016. PR Newswire. May 26, 2016. Accessed November 8, 2016. http://www.prnewswire.com/news-releases/precipitated-silica-to-account-for-70-volume-share-of-global-demand-for-speciality-silica-in-2016-future-market-insights-fmi-580992831.html.
5 Katz, H.S.; Milenewski, J.V. Handbook of Fillers for Plastics. Nostrand Reinhold Co., 1987.
6 Ciullo, P.A. Industrial Minerals and Their Uses: A Handbook and Formulary. Noyes Publishing, 1996.
7 Gysau, D. Fillers for Paints. Vincentz Network, 2011.
8 Michel-Sanchez, E. Impact of Particle Morphology on the Rheology of PCC-Based Coatings. Georgia Institute of Technology, 2005.
9 US Pat 6737467 B1 Decker and Sparks. E. I. Du Pont De Nemours and Co., 2000.
10 Pusheng Ting, A.; Luebbers, R.H. Viscosity of Suspensions of Spherical and Other Isodimensional Particles in Liquids. AIChE Journal volume 3, issue 1, 1957.
11 Mueller, S.; Llewellin, E.W.; Mader H.M. The Rheology of the Suspension of Solid Particles. Proceedings of The Royal Society A 2010 466. 1201-1228.
12 Bierwagen, G.P.; Hay, T.K. The Reduced Pigment Volume Concentration as an Important Parameter in Interpreting and Predicting the Properties of Organic Coatings. Progress in Organic Coatings volume 3, issue 4,281-303. Switzerland; Elsevier B.V., 1975.
13 Koleske, J.V. Paint and Coatings Testing Manual, Fourteenth Edition of the Gardner/Sward Handbook. ASTM Publications. ISBN 0-8031-2060-5, 1995.
14 Lee, S.M. Handbook of Composite Reinforcements. VCH Publishers, 1993.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!